Early results from a phase III trial support 30-minutes infusions of immunotherapy with some advantages for patients
As presented at the ESMO Asia Congress 2024 (Singapore, 6–8 December), preliminary findings from the single-arm phase IIIb TOURMALINE trial indicate that a 30-minute infusion time may be feasible with durvalumab in combination with gemcitabine-based chemotherapy for the first-line treatment of a patient population with advanced biliary tract cancer that reflects clinical practice (Abstract 133O).
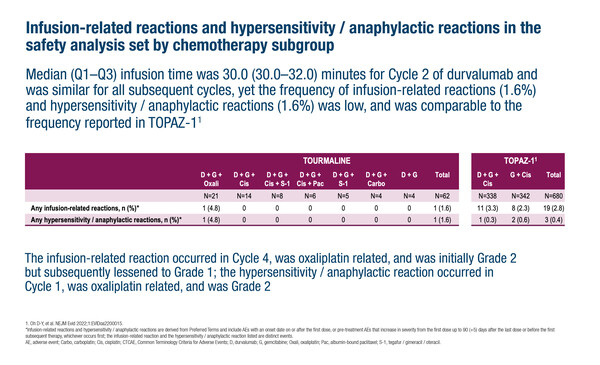
The analysis included 62 participants – of whom more than 90% were Asian – who received durvalumab 1500 mg (first infusion of 60 minutes then subsequent infusions of 30 minutes if tolerated) combined with an investigator-selected gemcitabine-based regimen. Only one patient had an infusion reaction adverse event (AE) following durvalumab and gemcitabine plus oxaliplatin during cycle 4, which was considered possibly related to oxaliplatin. Serious AEs (SAEs) were reported in 29.0% of patients, while 87.1% and 6.5% of patients, respectively, had an AE or SAE possibly related to any study treatment. Grade ≥3 AEs and SAEs were reported in 67.7% and 24.2% of patients, respectively, with no deaths related to AEs. Durvalumab dose delay due to AEs occurred in 25.8% of patients, with no patients experiencing durvalumab dose interruption or dose reduction, while 4.8% of patients discontinued any study treatment because of AEs. Immune-related AEs occurred in 29.0% of patients.
“The use of durvalumab is currently being optimised in the clinic also thanks to increasing experience in other tumour types, such as hepatocellular carcinoma and lung cancer,” explains Dr Lorenza Rimassa from the IRCCS Humanitas Research Hospital, Milan, Italy. “Strategies that make life easier for patients, here in terms of reduced clinic time, and for healthcare organisations, are advantageous but only if we have the data to support them,” she continues.
TOURMALINE builds on the TOPAZ-1 trial, which demonstrated that durvalumab plus chemotherapy significantly improved overall survival versus placebo plus chemotherapy in patients with untreated advanced biliary tract cancer (NEJM Evid. 2022;1:EVIDoa2200015). Unlike TOPAZ-1, which studied gemcitabine plus cisplatin only, TOURMALINE is evaluating a range of gemcitabine-based regimens combined with durvalumab (gemcitabine alone or in combination with oxaliplatin, carboplatin, cisplatin, tegafur-gimeracil-oteracil [S-1], cisplatin plus S-1 or cisplatin plus nab-paclitaxel). TOPAZ-1 included only patients with World Health Organization/Eastern Cooperative Oncology Group performance status of 0 or 1, while around one-quarter (24.2%) of patients in TOURMALINE had performance status of 2 at baseline.
“Phase III trials are important to gain regulatory approval but studies such as TOURMALINE are needed to generate data in populations that more closely reflect clinical practice and the final results are eagerly awaited,” notes Rimassa. “Another example is the phase IIIb SIERRA trial (Ann Oncol. 2023;34(Suppl 4):S1554), which is assessing tremelimumab plus durvalumab in a broader population of patients with unresectable hepatocellular carcinoma than the initial HIMALAYA trial (NEJM Evid. 2022;1:EVIDoa2100070). Such trials are extremely valuable to inform clinical practice and I hope to see others like these in the future.”
Programme details
Oh D-Y, et al. Safety of 30 min infusion of durvalumab (D) in combination with gemcitabine (G)-based chemotherapy in first-line treatment (tx) of advanced biliary tract cancer (aBTC): TOURMALINE early results. ESMO Asia Congress 2024, Abstract 133O
Proffered Paper Session – Gastrointestinal tumours, 06.12.2024, h. 13:45 – 15:15, Hall 406