New subpopulation analyses from three international phase III trials confirm efficacy of immune checkpoint inhibitors across geographical areas
Full-population analyses of three phase III trials of first-line checkpoint inhibitor therapies – the RATIONALE-301, RATIONALE-306 and HIMALAYA studies – have previously reported that they reached their primary endpoints, showing outcome benefits for immunotherapy in the first-line setting of unresectable hepatocellular carcinoma (HCC) and oesophageal squamous cell carcinoma (ESCC) (Ann Oncol. 2022;33[Suppl 7]:S808–S869; Ann Oncol. 2022;33[Suppl 4]:S375; NEJM Evid 2022;1:8). These benefits have now been confirmed for Asian patients, as data presented at ESMO Asia Congress 2022 (Singapore, 2-4 December 2022) report.
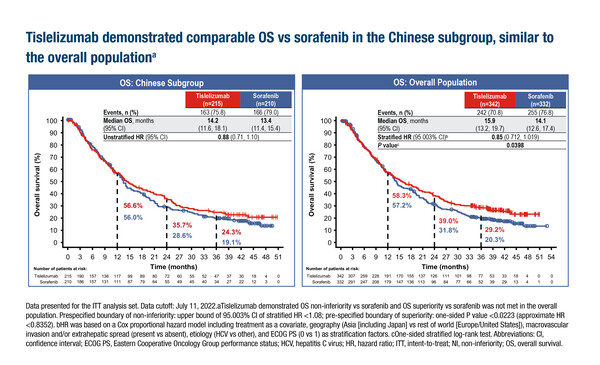
The RATIONALE-301 and RATIONALE-306 trials investigated the use of the PD-1 inhibitor tislelizumab in unresectable hepatocellular carcinoma (HCC) and ESCC, respectively. In the Chinese subpopulation analysis of RATIONALE-301 (n=425), the overall survival (OS) for tislelizumab was longer than that for sorafenib, with medians of 14.2 months (95% confidence interval [CI] 11.6–18.1) and 13.4 months (95% CI 11.4–15.4), respectively (LBA2). OS times in the overall population (n=674) were 15.9 months (95% CI 13.2–19.7) for tislelizumab and 14.1 months (95% CI 12.6–17.4) for sorafenib. Results for objective response rate (ORR), duration of response (DoR), progression-free survival (PFS) and adverse event profiles were also comparable between the full-population and Chinese subpopulation analyses.
In the RATIONALE-306 trial, the Asian subpopulation analysis (n=486) reported median OS times for tislelizumab plus chemotherapy (platinum in combination with fluoropyrimidine or paclitaxel) and chemotherapy alone of 18.3 months and 11.5 months, respectively (unstratified hazard ratio [HR] 0.67; 95% CI 0.54–0.84) and median PFS times of 7.2 months versus 5.6 months (unstratified HR 0.62; 95% CI 0.50–0.76) (Abstract 70O). These results reflect those of the full-population interim analysis (n=649), which reported median OS times of 17.3 months for tislelizumab plus chemotherapy versus 10.6 months with chemotherapy alone (HR 0.66; 95% CI 0.54–0.80; p<0.0001) (Ann Oncol. 2022;33[Suppl 4]:S375). Addition of tislelizumab was also associated with a higher ORR and a longer median DoR than chemotherapy alone in the Asian subpopulation analysis.
In the HIMALAYA trial, the Asian subpopulation analysis (including Hong Kong, India, South Korea, Taiwan, Thailand and Vietnam, but not Japan) (n=479) revealed that median OS times for the Single Tremelimumab Regular Interval Durvalumab (STRIDE) regimen (16.5 months; 95% CI 12.6–22.1; HR 0.68) and for durvalumab (16.6 months; 95% CI 12.2–19.2; HR 0.83) were longer than for sorafenib (11.8 months; 95% CI 9.4–14.7) (Abstract 67O). Corresponding median OS times in the overall population (n=1,171) were 16.4 months (95% CI 14.2–19.6; HR 0.78) for STRIDE, 16.6 months (95% CI 14.1–19.1; HR 0.86) for durvalumab and 13.8 months (95% CI 12.3–16.1) for sorafenib. Also, results from an additional subpopulation analysis of patients from Hong Kong/Taiwan (n=141) were generally consistent with the overall Asian subpopulation analysis, with median OS times of 29.4 months (HR 0.44) for STRIDE, 23.6 months (HR 0.64) for durvalumab and 19.1 months for sorafenib.
“When we talk about immunotherapy, it is important to confirm that we have similar activity in different populations around the world. This is mainly because we are still learning about immunotherapy and the factors that can impact on its activity,” says Dr Angela Lamarca from Fundación Jiménez Díaz University Hospital, Madrid, Spain. “The results from these Asian subpopulation analyses provide confirmation that treatment with immune checkpoint inhibitors is effective across ethnicities. The consistency of the results across ethnicities also demonstrates that the practice of duplicating European trials in Asia is no longer necessary and that worldwide studies should continue to be pursued.”
Commenting on the study results, Lamarca says: “In the RATIONALE studies, the Asian subpopulations represent a majority of the overall populations – 63% in RATIONALE-301 and 75% in RATIONALE-306 – so it is perhaps not surprising that these subpopulation analyses are consistent with the overall analyses. Indeed, it might be interesting to see the efficacy results in the non-Asian subpopulations. In the HIMALAYA trial, however, the Asian subpopulation represents a minority of the overall population – only 41% – and so the results from this analysis are particularly reassuring in confirming the benefits of immunotherapy across ethnicities. It is also encouraging to see that the 29% of patients of Chinese descent within the Asian subpopulation had similar outcomes to the entire Asian subpopulation.”
The results also give some insights into the disease aetiology of HCC. “It has been suggested that the aetiology of HCC could be a relevant biomarker for immunotherapy efficacy,” explains Lamarca. “Viral hepatitis is generally more prevalent in Asia and non-viral aetiologies are usually more prevalent outside Asia. The results from the Asian subpopulation analyses of the RATIONALE-301 and the HIMALAYA trials support that treatment with immune checkpoint inhibitors is likely to be effective across viral and non-viral aetiologies,” she says.
Abstracts discussed:
Qin S, et al. Tislelizumab (TIS) versus sorafenib (SOR) in first-line (1L) treatment of unresectable hepatocellular carcinoma (HCC): the RATIONALE-301 Chinese subpopulation analysis. ESMO Asia Congress 2022, LBA2
Mini Oral Session: Gastrointestinal tumours 04.12.2022, h. 09:15 – 10:45, Hall 404. Also watch the session on the Congress virtual platform.
Kato K, et al. Randomized, global, Phase 3 study of tislelizumab (TIS) + chemotherapy (chemo) vs chemo as first-line (1L) therapy for advanced or metastatic esophageal squamous cell carcinoma (ESCC) (RATIONALE-306): Asia subgroup. ESMO Asia Congress 2022, Abstract 70O
Proffered Paper Session: Gastrointestinal tumours 02.12.2022, h. 16:15 – 17:45, Hall 406. Also watch the session on the Congress virtual platform.
Chan SL, et al. Outcomes in the Asian subgroup of the Phase 3 HIMALAYA study of tremelimumab (T) plus durvalumab (D) in unresectable hepatocellular carcinoma (uHCC). ESMO Asia Congress 2022, Abstract 67O
Proffered Paper Session: Gastrointestinal tumours 02.12.2022, h. 16:15 – 17:45, Hall 406. Also watch the session on the Congress virtual platform.