Positive results in intermediate and advanced disease come after the failure of global trials investigating the combination of TACE with targeted agents
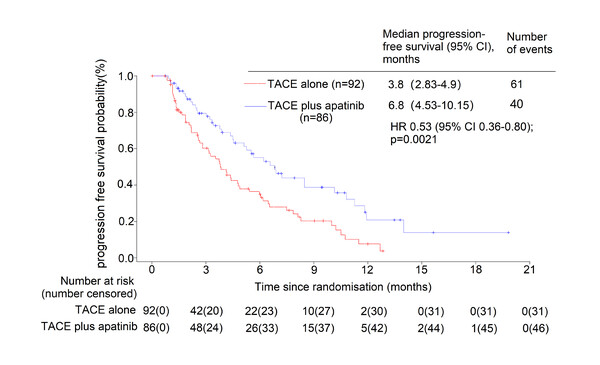
Following negative results in global trials studying tyrosine kinase inhibitors plus transarterial chemoembolisation (TACE), findings from a study presented at the ESMO Asia Congress 2022 (Singapore, 2–4 December) now show the combination of TACE with apatinib prolonged progression-free survival (PFS) in a Chinese population with intermediate and advanced hepatocellular carcinoma (HCC) (Abstract 66O). In the prospective, multicentre, randomised trial involving 178 patients, the primary endpoint of median PFS was significantly longer with TACE plus apatinib (6.83 months) than with TACE alone (3.81 months; hazard ratio 0.53; 95% confidence interval [CI] 0.36–0.80; p=0.0021). Median overall survival (OS) was not evaluable (NE) (95% CI 13.4–NE) in patients who received TACE plus apatinib and 18.2 months (95% CI 12.3–NE) for patients who received TACE alone. Furthermore, objective response and disease control rates were higher with the combination than TACE alone (30.23% versus 23.91% and 70.93% versus 61.96%).
These results contrast findings previously reported in similar trials such as the global phase II SPACE trial (J Hepatol. 2016;64:1090–1098) and the UK phase III TACE 2 trial (Lancet Gastroenterol Hepatol. 2017;2:565–575) where sorafenib in combination with TACE did not improve PFS compared with TACE alone. “The positive findings of the Chinese study may be specific for apatinib, which is rarely used outside of China, or may be due to regional variation, such as the different aetiology of hepatocellular carcinoma in Chinese patients,” suggests Dr Richard Hubner from the Christie NHS Foundation Trust, Manchester, UK, commenting on the results presented. He points out that PFS in the TACE alone arm was lower than expected, which may have occurred as a result of the study population’s baseline risk factors. The timing of the systemic treatment in relation to TACE also varied. He notes, “In TACE 2 and SPACE, sorafenib was initiated before TACE, aiming to maximise the anti-angiogenic effect, but in the current study, apatinib was given after the procedure.” Although no new safety signals were identified with apatinib, Hubner raises concerns that the doubling in grade ≥3 AEs in the TACE plus apatinib group compared to the TACE alone group (16.5% versus 8.6%). may be problematic if it impacts on patients’ quality of life.
Further study of the apatinib-TACE combination in a large phase III trial may help to confirm the findings in light of previous trials. According to Hubner, results are now also eagerly awaited from several ongoing studies investigating the combination of immune checkpoint inhibition with TACE, such as the phase II/III TACE-3 trial assessing nivolumab in combination with TACE (NCT04268888) and the phase III EMERALD-1 trial of durvalumab and bevacizumab with TACE (NCT03778957).
Abstract discussed:
Liang B, et al. TACE combined with apatinib in patients with intermediate and advanced hepatocellular carcinoma: A prospective, multi-center, randomized clinical trial. ESMO Asia Congress 2022, Abstract 66O
Presidential Symposium 03.12.2022, h. 10:45 – 12:15, Hall 406. Also watch the session on the Congress virtual platform