Results from the RUBY trial show improvements both in progression-free survival and overall survival in patients treated with dostarlimab plus standard chemotherapy, indicating a new standard of care
Data presented at the ESMO Virtual Plenary in March 2023 provided the first confirmation that adding immunotherapy to standard chemotherapy for first-line treatment of advanced or first recurrence of endometrial cancer significantly improves progression-free survival (PFS) compared to chemotherapy alone, with a promising early indication of improved overall survival (OS) (Abstract VP2-2023).
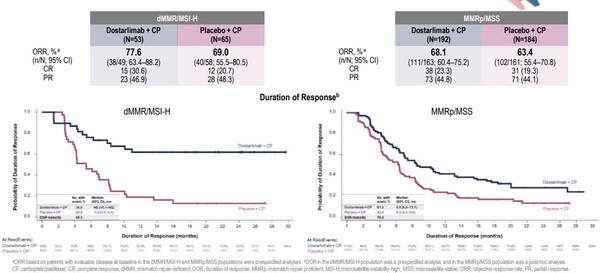
In the placebo-controlled randomised phase III RUBY trial (ENGOT-EN6-NSGO/GOG3031), clinical benefits were higher in patients with ‘hot’ endometrial tumours that have deficient DNA mismatch repair mechanisms (dMMR) and were maintained for at least two years. Of 494 patients randomised, 23.9% had dMMR/ high microsatellite instability (MSI-H) tumours, 47.8% had recurrent disease, 18.6% and 33.6% had primary stage III and IV disease, respectively.
Overall median PFS was 11.8 months in patients treated with the PD-1 inhibitor dostarlimab, and carboplatin plus paclitaxel, compared to 7.9 months in those treated with standard chemotherapy and placebo (hazard ratio [HR] 0.64; confidence interval [CI] 0.507-0.800, p<0.0001). In patients with ‘hot’ tumours with dMMR or MSI-H, PFS could not be estimated in the immunotherapy+chemotherapy group because too few cancers progressed during the 25-month follow-up. In contrast, PFS was 7.7 months in the chemotherapy+ placebo group (HR 0.28; CI 0.162-0.495, p<0.0001). An exploratory analysis of results in patients with ‘cold’ tumours that were mismatch repair proficient (MMRp) or microsatellite stable (MSS) showed PFS of 9.9 months and 7.9 months, respectively (HR 0.76; CI 0.592-0.981, NA).
Investigators also observed an early trend in OS in favour of combination treatment. In the immunotherapy+ chemotherapy group, 71.3% of patients were alive at 24 months compared to 56% in the chemotherapy+placebo group. Among those with ‘hot’ tumours, OS was 83.3% and 58.7% respectively. In those with ‘cold’ tumours, OS was 67.7% and 55.1% respectively.
According to Dr Ilaria Colombo, Oncology Institute of Southern Switzerland, Bellinzona, Switzerland, the results of this trial show a new standard of care for women with advanced or relapsed endometrial cancer. “We now need to see the results of other trials using different types of immunotherapy, and also to find out the best duration of maintenance immunotherapy and whether immunotherapy is more effective than chemotherapy in patients with mismatch repair deficiency or microsatellite instability, or if it could be used without chemotherapy,” she said. “There was also a clinical benefit in patients without these DNA repair deficiencies, though of smaller magnitude, and we still need to identify better treatment options for patients with mismatch proficient or microsatellite stable tumours that represent about 70% of patients with advanced or recurrent endometrial cancer. The trend in overall survival benefit is also encouraging, but this needs to be confirmed with longer follow-up, taking into account that in the standard arm many patients received immunotherapy in subsequent lines of treatment.”
In the study, no unexpected safety issues with the combination of immunotherapy and chemotherapy were reported, and there was evidence of improved quality of life for patients. “The beneficial effect on quality of life is important because we need to know that patients are not only living longer but that they are also living better, especially when we use maintenance therapy and patients remain on treatment for a long time.” concludes Colombo.
Study results were published simultaneously with data presentation in The New England Journal of Medicine.
Abstract discussed
M.R. Mirza et al. Dostarlimab+chemotherapy for the treatment of primary advanced or recurrent (A/R) endometrial cancer (EC): A placebo (PBO)-controlled randomised phase III trial (ENGOT-EN6-NSGO/GOG-3031/RUBY) (VP2-2023). ESMO Virtual Plenary, 27 March 2023.
Watch the abstract presentation on OncologyPRO.