Extended analyses from two regulatory phase III trials show that the benefits of anti-PD-1-based therapy persist long term, even in patients with brain metastases
As presented at the European Lung Cancer Congress (ELCC) 2023 (Copenhagen, 29 March–1 April), updated data from the EMPOWER-Lung 1 (Lancet. 2021;397:592–604) and EMPOWER-Lung 3 (Nat Med. 2022;28:2374–2380) trials confirm a durable activity of the immunotherapeutic, cemiplimab, in non-small cell lung cancer (NSCLC) at advanced disease stages and without a targetable driver.
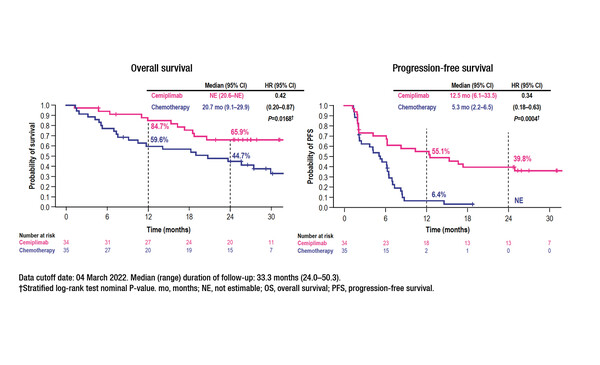
An early subgroup analysis of the EMPOWER-Lung 1 trial indicated that the survival benefits of first-line treatment with cemiplimab monotherapy in patients with advanced NSCLC and PD-L1 expression ≥50% may extend, at least in the short-term, to patients with clinically stable brain metastases (J Clin Oncol. 2021;39(Suppl 15):9085–9085). According to new data from a post-hoc analysis, at a median follow-up of 33.3 months (range 24.0–50.3) among patients with clinically stable brain metastases at randomisation, cemiplimab (n=34) prolonged median overall survival (OS) (not estimable versus 20.7 months; hazard ratio [HR] 0.42; 95% confidence interval [CI] 0.20–0.87; p=0.0168) and median PFS (12.5 months versus 5.3 months; HR 0.34; 95% CI 0.18–0.63; p=0.0004) compared with chemotherapy (n=35) (Abstract 10MO). Cemiplimab also led to a higher objective response rate (55.9% versus 11.4%) (odds ratio 9.27; 95% CI 2.62–32.74; p=0.0002), a longer median duration of response (DoR) (31.7 months versus 12.5 months) and a lower rate of post-baseline brain-specific disease progression (14.7% versus 20.0%) than chemotherapy. In addition, there were fewer grade ≥3 treatment-emergent adverse events with cemiplimab than with chemotherapy (35.3% versus 60.0%).
A second presentation at ELCC 2023 provided positive data from an additional 12- month follow-up of the EMPOWER-Lung 3 trial in patients with NSCLC and any level of PD-L1 expression (Nat Med. 2022;28:2374–2380). Researchers confirmed the durability of survival improvements with cemiplimab plus chemotherapy versus chemotherapy alone, with median OS times of 21.1 months versus 12.9 months, respectively (HR 0.65; 95% CI 0.51–0.82; p=0.0003), at a median follow-up of 28.4 months (Abstract 5O). Median PFS times were 8.2 months with cemiplimab plus chemotherapy and 5.5 months with chemotherapy alone (HR 0.55; 95% CI 0.44–0.68; p<0.0001). Objective response rates (43.6% versus 22.1%) and DoR (16.4 and 7.3 months) also favoured cemiplimab. Grade ≥3 TEAEs were reported in 48.7% of patients receiving cemiplimab plus chemotherapy and 32.7% receiving chemotherapy.
“The data presented are reassuring. Cemiplimab is confirmed as an important option for the treatment of NSCLC without a targetable driver – it is particularly valuable because, unlike some other checkpoint inhibitors, its label includes locally advanced disease, which cannot be treated with definitive chemoradiotherapy, not just metastatic NSCLC,” says Assistant Prof. Lizza Hendriks from Maastricht University, Netherlands. “Although the numbers of patients with brain metastases included in the EMPOWER-Lung 1 trial analysis are small, meaning we cannot draw firm conclusions, the efficacy data are in line with what has been shown in a pooled analysis of pembrolizumab in a similar patient population with high PD-L1-expressing NSCLC and brain metastases (JTO Clin Res Rep. 2021;2:100205). This not only supports the use of cemiplimab in this patient group but also reinforces the view that stable brain metastases should not be a reason to exclude patients from immunotherapy.”
Moving forward, Hendriks would like to see more trials including patients with asymptomatic and untreated brain metastases. “Screening for brain metastases is now recommended by management guidelines, such as the EANO–ESMO Clinical Practice Guidelines (Ann Oncol. 2021;32:1332–1347), so we can expect to see increasing numbers of patients with asymptomatic brain metastases and we need to know how to treat them,” she says. “Data for immunotherapy monotherapy, which come mainly from single-arm studies or retrospective analyses, suggest that some patients achieve durable benefit from this treatment, but there is also a risk of intracranial progression with this approach. However, upfront metastases-directed radiotherapy has the potential for long-term toxicities. We really need a randomised controlled trial comparing upfront radiotherapy followed by immunotherapy versus upfront immunotherapy followed by radiotherapy for disease progression.”
Abstracts discussed:
Kilickap S, et al. EMPOWER-Lung 1: Cemiplimab (CEMI) monotherapy as first-line (1L) treatment of patients (pts) with brain metastases from advanced non-small cell lung cancer (aNSCLC) with programmed cell death-ligand 1 (PD-L1) ≥50% — 3-year update. European Lung Cancer Congress 2023, Abstract 10MO
Mini Oral 2, 31.03.2023, h. 08:15 – 09:15, Auditorium 4
Makharadze T, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: Longer follow-up results from the phase III EMPOWER-Lung 3 trial. European Lung Cancer Congress 2023, Abstract 5O
Proffered Paper 2, 30.03.2023, h. 15:10 – 16:40, Auditorium 1