Phase III data show significant survival improvements with the TROP2-directed antibody–drug conjugate after progression on EGFR tyrosine kinase inhibitors
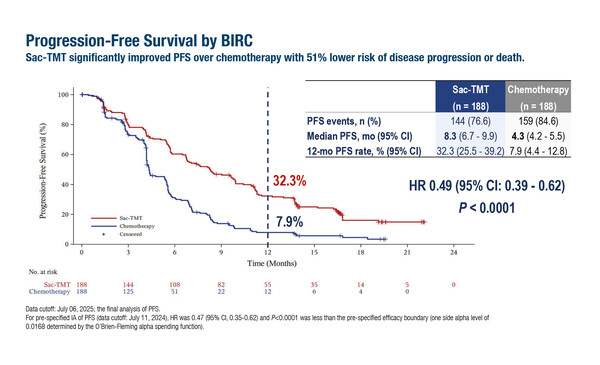
According to results presented in today's Presidential Symposium at the ESMO Congress 2025 (Berlin, 17–21 October), the TROP2-directed antibody–drug conjugate (ADC) sacituzumab tirumotecan significantly prolonged median progression-free survival (PFS) compared with chemotherapy (8.3 months versus 4.3 months; hazard ratio [HR] 0.49; 95% confidence interval [CI] 0.39–0.62; p<0.0001) in 376 patients with EGFR-mutated non-small cell lung cancer (NSCLC) and prior EGFR tyrosine kinase inhibitor (TKI) therapy (LBA5). In an interim analysis of overall survival (OS) at a median follow-up of 18.9 months, sacituzumab tirumotecan reduced the risk of death by 40% (HR 0.60; 95% CI 0.44–0.82; p=0.0006), with median OS not yet reached compared with 17.4 months for chemotherapy.
These results from the OptiTROP-Lung04 trial confirm findings from an earlier phase II trial (BMJ 2025;389:e085680) and, “deliver the first meaningful survival gains in a population where, historically, treatment options have been limited to chemotherapy and have resulted in only modest benefits,” highlights Prof. Antonio Passaro from the European Institute of Oncology, Milan, Italy. The current treatment landscape in this setting is rapidly expanding, with several emerging options beyond chemotherapy alone. Based on the MARIPOSA-2 trial (Ann Oncol. 2024;35:77–90), the combination of amivantamab plus chemotherapy has become the new global standard of care. In parallel, trial data have led to the approval of regimens including the bispecific antibody, ivonescimab (JAMA. 2024;332:561–570) in China and the TROP2-directed ADC, datopotamab deruxtecan (Dato-DXd) (J Thorac Oncol. 2025:S1556-0864(25)00761-0) in the United States for patients progressing after EGFR TKI therapy.
In the OptiTROP-Lung04 trial, all efficacy endpoints – including PFS, OS and overall response rate – showed a significant improvement in favour of sacituzumab tirumotecan, confirming its meaningful clinical activity in this population. Nevertheless, a subset of patients appeared to derive limited benefit from treatment, underscoring the need for further biomarker-driven refinement of patient selection. “As with other ADCs, resistance mechanisms may emerge, so future combination or sequential strategies will likely be needed to prolong benefit,” notes Passaro. Another important area for investigation is intracranial efficacy, given the high incidence of brain metastases in EGFR-mutated disease. “Assessing activity within the central nervous system will be essential to guide optimal sequencing,” he adds.
The safety findings demonstrate that sacituzumab tirumotecan was well tolerated with no unexpected or clinically limiting adverse events. The most common toxicities in the ADC arm were mild-to-moderate haematological and mucosal events, with no treatment-related adverse events leading to discontinuation or death.
Given that available data for sacituzumab tirumotecan originate entirely from Chinese studies, broader validation is now needed. The global phase III TroFuse-009 trial comparing sacituzumab tirumotecan to platinum-based doublet chemotherapy in a similar population as in OptiTROP-Lung04 (J Thor Oncol. 2024;19:S246–247) is ongoing and will provide additional details about the efficacy, safety and long-term outcomes of the ADC across diverse clinical backgrounds.
The post-EGFR TKI setting in NSCLC remains a rapidly evolving field, with several ADCs in advanced development. Findings from OptiTROP-Lung04 were consistent with analyses of Dato-DXd (J Thorac Oncol. 2025:S1556-0864(25)00761-0). “Results for both agents confirm that TROP2 is a robust target for a biomarker-agnostic therapeutic strategy after EGFR TKI progression,” says Passaro. The global phase III TROPION-Lung15 trial is currently investigating Dato-DXd with or without osimertinib versus platinum doublet chemotherapy (J Thor Oncol. 2024;20:S87–88). In addition, Passaro notes, “EGFR/HER3- and MET-targeted ADCs are also undergoing clinical investigation and have produced encouraging early efficacy signals in the EGFR-resistant setting. If these results are confirmed in ongoing phase III studies, they are expected to redefine the management of resistance to EGFR TKIs, particularly osimertinib, marking the beginning of a new therapeutic era for EGFR-mutated NSCLC.”
Programme details:
Zhang L, et al. Sacituzumab tirumotecan (sac-TMT) vs platinum-based chemotherapy in EGFR-mutated (EGFRm) non-small cell lung cancer (NSCLC) following progression on EGFR-TKIs: results from the randomized, multi-center phase 3 OptiTROP-Lung04 study. ESMO Congress 2025 - LBA5