Negative results are reported in SKYSCRAPER-03, while induction immunochemotherapy is investigated as a potential novel approach for unresectable stage II NSCLC
Since the PACIFIC trial in 2017, which set the current standard of care for patients with unresectable stage III non-small cell lung cancer (NSCLC) – concurrent chemoradiotherapy followed by the PD-L1 inhibitor durvalumab (N Engl J Med. 2017;377:1919–1929) – attempts to improve outcomes with concurrent immunotherapy or dual blockade have not succeeded. Similarly, data on novel treatment strategies from two trials presented at the ESMO Congress 2025 (Berlin, 17–21 October) demonstrate non-practice-changing value.
Combination treatment with the PD-L1 inhibitor atezolizumab plus the T cell immunoreceptor with Ig and ITIM domains (TIGIT) inhibitor tiragolumab did not improve outcomes compared with durvalumab in the phase III SKYSCRAPER-03 trial of 829 patients with unresectable, stage III NSCLC without progression after concurrent chemoradiotherapy (LBA69, key results in the box below). No benefits were seen for the combination compared with durvalumab in terms of the primary endpoint, median progression-free survival (mPFS) in all-comers (14.2 months versus 13.8 months, respectively) or in PD-L1 positive patients (19.4 months versus 16.6 months, respectively), or in terms of median overall survival (all-comers: 45.6 months versus 45.8 months, respectively; PD-L1 positive: not reached versus 54.8 months, respectively). The safety profile of atezolizumab plus tiragolumab was consistent with previous reports.
Commenting on these findings, Prof. Corinne Faivre-Finn from the University of Manchester and The Christie NHS Foundation, Manchester, UK, notes, “Although the phase II CITYSCAPE study had previously indicated that dual blockade of PD-L1 and TIGIT could enhance antitumour immune responses in the metastatic NSCLC setting (Lancet Oncol. 2022;23:781–792), a subsequent phase III study, -SKYSCRAPER-01, failed to demonstrate survival or PFS benefits of tiragolumab added to atezolizumab compared to atezolizumab plus placebo. SKYSCRAPER-03 reaffirms these results in the setting of stage III NSCLC. Interestingly, outcomes for patients in the control arm of SKYSCAPER-03 were comparable to those in the experimental arm of the PACIFIC trial, reinforcing that concurrent chemoradiotherapy followed by durvalumab should remain the standard of care for patients with unresectable NSCLC.” She also emphasises the need for better patient stratification: “The lack of reliable biomarkers in stage III NSCLC is a significant challenge. Improved biomarkers are urgently needed to identify subgroups of patients who might benefit from these novel treatment regimens.”
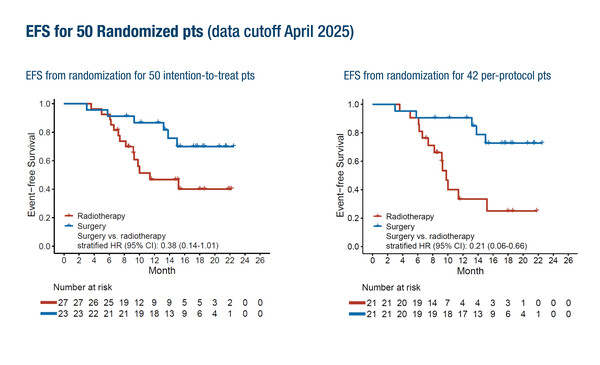
Encouraging early findings were reported from the open-label phase II LungMate-013 study, where 100 patients with unresectable stage III NSCLC initially received induction therapy with the anti-PD1 antibody serplulimab plus chemotherapy, and those who converted to resectable disease according to local assessment were subsequently randomised to surgery or radiotherapy (Abstract 1818MO, key results in the box below). Among randomised patients (n=50), the hazard ratio (HR) for surgery versus radiotherapy in terms of event-free survival (EFS) indicated a trend in favour of surgery in the intention-to-treat population (stratified HR 0.38; 95% confidence interval [CI] 0.14–1.01). This effect was more pronounced in the per-protocol group (n=42). No new safety signals were reported with this treatment approach.
Faivre-Finn welcomed the study as an important step forward in exploring the role of induction immunochemotherapy in these patients. “Randomised studies like this are exactly what the field needs, as they address the critical question of whether induction therapy can convert patients with more advanced disease to operable status,” she says. At the same time, she notes that interpretation of the findings is challenging. “The trial is very small and it is also unclear how resectability was defined or how patients were restaged after induction. Another concern is that some patients may miss the opportunity for curative-intent treatment if they progress or develop toxicity during induction.” She explains, however, that the study highlights a key area of ongoing debate in thoracic oncology and underscores the need for larger, rigorously designed trials to define the optimal management strategy in this setting.
She further highlights that the management of unresectable stage III NSCLC remains challenging, as not all patients are suitable for the current standard treatment. Many are elderly and/or have significant comorbidities, making them ineligible for the concurrent administration of chemotherapy with radiotherapy followed by immunotherapy, and leaving many to receive radiotherapy alone. Research in this field is now investigating novel agents, such as in the phase Ib CONCORDE study (Clin Transl Radiat Oncol. 2020;25:61–66), with the aim of developing chemotherapy-free multimodality approaches.
“Future progress will depend on better patient selection, biomarker development and strategies for patients unfit for current standard treatments. Uniform definitions of resectability, such as those proposed by the European Organisation for Research and Treatment of Cancer (EORTC) Lung Cancer Group, will be essential for comparing trial results and guiding clinical decisions,” concludes Faivre-Finn.
At a glance:
Dziadziuszko R, et al. SKYSCRAPER-03: Phase 3, open-label, randomised study of atezolizumab (atezo) + tiragolumab (tira) vs durvalumab (durva) in locally advanced, unresectable, stage III non-small cell lung cancer (NSCLC) after platinum-based concurrent chemoradiation (CCRT). ESMO Congress 2025 - LBA69
- PD-L1 all-comers: N=829 (atezolizumab + tiragolumab: n=413; durvalumab: n=416)
- PD-L1+: N=419 (atezolizumab + tiragolumab: n=209; durvalumab: n=210)
- Atezolizumab + tiragolumab vs durvalumab:
- mIRF-PFS (all-comers): 14.2 mo vs 13.8 mo (stratified HR 1.00; 95% CI 0.84−1.19)
- mIRF-PFS (PD-L1+): 19.4 mo vs 16.6 mo; (stratified HR 0.96; 95% CI 0.75–1.23; p=0.7586)
- mOS (all-comers): 45.6 mo vs 45.8 mo (stratified HR 0.98; 95% CI 0.80−1.20)
- mOS (PD-L1+): NE vs 54.8 mo (stratified HR 0.99; 95% CI 0.73–1.34)
- Grade 3–4 TRAEs: 13.8% vs 10.7%
- AEs leading to treatment withdrawal: 15.5% vs 9.0%
Wang S, et al. Surgery versus radiotherapy after induction therapy with serplulimab combined with chemotherapy for unresectable stage IIIB-IIIC non-small cell lung cancer: A randomized controlled, open-label, phase II trial. ESMO Congress 2025 - Abstract 1818MO
- N=50 (surgery: n=23; radiotherapy: n=27)
- Surgery vs radiotherapy:
- EFS (ITT population, n=50): stratified HR 0.38; 95% CI 0.14–1.01
- EFS (per-protocol population, n=42): stratified HR 0.21; 95% CI 0.06–0.66
- Grade ≥3 TRAEs (induction therapy): 58%
- AEs leading to death (induction therapy): 2%