Data from the ELEVATE and ALINA studies highlight the gains provided by adjuvant ALK therapy in patients with ALK-positive disease
The paradigm shift brought by alectinib in ALK-positive early-stage non-small cell lung cancer (NSCLC) (N Engl J Med. 2024;390:1265–1276) continues with ensartinib, which demonstrated clinical activity following adjuvant chemotherapy in the double-blind phase III ELEVATE trial. Data presented at the ESMO Congress 2025 (Berlin, 17–21 October) further support the integration of ALK tyrosine kinase inhibitors (TKIs) in this setting.
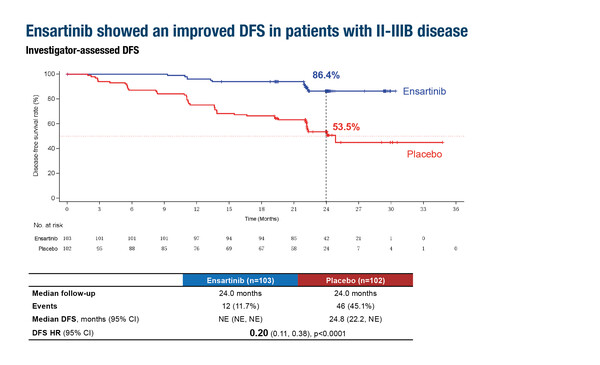
In a pre-specified interim analysis of the ELEVATE trial, adjuvant ensartinib following chemotherapy resulted in an 80% reduction in the risk of disease recurrence compared with placebo in patients with completely resected, stage IB–IIIB ALK-positive NSCLC. Disease-free survival (DFS), the primary endpoint, was associated with a hazard ratio (HR) of 0.20 in patients with stage II–IIIB disease (95% confidence interval [CI] 0.11–0.38; p<0.0001) and in the intent-to-treat (ITT) population (95% CI 0.10–0.37; p<0.0001) (LBA66, key results in the box below). Ensartinib also improved the 2-year DFS rate compared with placebo in both populations “These data position ensartinib as a potential new treatment option in the adjuvant setting,” comments Prof. Marcello Tiseo from the University Hospital of Parma, Italy. “A significant reduction in central nervous system (CNS) recurrences is also of particular relevance given the high incidence of brain metastases in ALK-positive NSCLC,” he adds. The safety profile appeared to be consistent with previous reports, suggesting manageable tolerability in the context of adjuvant treatment.
While additional analyses will be essential to determine the durability of benefit, potential overall survival (OS) advantage, and long-term safety of ensartinib, DFS data are similar to those reported with the standard of care, alectinib, in the phase III, randomised ALINA study, where 2-year DFS was 93.8% versus 63.0% with chemotherapy (HR 0.24) (N Engl J Med. 2024;390:1265–1276). However, the ELEVATE trial also provides the first evidence of clinical activity of an ALK TKI after chemotherapy in early-stage ALK-positive NSCLC. “This is different to the ALINA trial where alectinib was compared directly with adjuvant chemotherapy,” Tiseo explains. Other differences between the studies include enrolment of an all-Asian population in ELEVATE, compared with ~55% Asian patients in ALINA, and staging by the American Joint Committee on Cancer (AJCC) 8th edition in ELEVATE versus use of the AJCC 7th edition in ALINA.
Also presented at the Congress were updated results from the ALINA trial in 257 patients with stage IB–IIIA ALK-positive NSCLC (Abstract 1787MO, key results in the box below). “With nearly 4 years of follow-up, alectinib continues to demonstrate a robust and durable DFS benefit over chemotherapy that was largely consistent across all subgroups,” observes Tiseo. Sustained reductions in the risk of disease recurrence were observed, with HRs of 0.36 and 0.35 in stage II–IIIA and overall ITT populations, respectively, while a clinically meaningful CNS-DFS benefit was maintained in the ITT population (HR 0.37; 95% CI 0.19–0.74). The 4-year OS rates were 98.4% with alectinib and 92.4% with chemotherapy. “OS data are still immature, but the magnitude and durability of DFS improvement, coupled with a 4-year OS rate of almost 100% and a good safety profile, reinforce the role of alectinib as standard of care for resected ALK-positive NSCLC and also highlight the therapeutic progress made in this field over recent years,” he remarks.
The comparable 2-year DFS rates reported in the ELEVATE and ALINA studies raise doubts over the role of adjuvant chemotherapy in early-stage ALK-positive NSCLC. Tiseo suggests, “A more mature follow-up period will be useful to confirm the long-term benefits of chemotherapy followed by ensartinib versus TKI alone after surgery. Adjuvant chemotherapy may have a place in the treatment of patients with ALK-positive NSCLC who are at highest risk of recurrence, but currently the data indicate that alectinib remains the best option in this setting.”
Both studies provide a good rationale for investigating novel, third- and fourth-generation TKIs in earlier-stage ALK-positive NSCLC, including as peri-operative treatment. “Agents such as the third-generation ALK TKI lorlatinib (which has demonstrated prolonged progression-free survival in patients with advanced NSCLC in the phase III CROWN study; J Clin Oncol. 2024;42:3400–3409), and fourth-generation TKIs, NVL-655 (neladalkib) and TPX-0131 (zotizalkib), may have the potential to further extend survival in resected patients,” Tiseo concludes.
At a glance:
Yue D, et al. Ensartinib as adjuvant therapy in patients (pts) with stage IB–IIIB ALK-positive (ALK+) non-small cell lung cancer (NSCLC) after complete tumor resection: the phase III randomized ELEVATE trial. ESMO Congress 2025 - LBA66
- N=274 (ensartinib n=137; PBO n=137)
- Ensartinib vs PBO (stage II–IIIB), median follow-up 24.0 mo (both arms):
- DFS HR 0.20 (95% CI 0.11–0.38; p<0.0001)
- 2-year DFS rate: 86.4% vs 53.5%
- Ensartinib vs PBO (ITT; stage IB–IIIB), median follow-up 22.2 mo vs 22.1 mo:
- DFS HR 0.20 (95% CI 0.10–0.37; p<0.0001)
- 2-year DFS rate: 87.3% vs 57.2%
- Grade ≥3 TEAE: 35.8% vs 18.2%
Dziadziuszko R, et al. Updated results from the phase III ALINA study of adjuvant alectinib vs chemotherapy (chemo) in patients (pts) with early-stage ALK+ non-small cell lung cancer (NSCLC). ESMO Congress 2025 - Abstract 1787MO
- N=257 (alectinib n=130; chemo n=127)
- Median follow-up: 48.0 mo (alectinib) and 47.4 mo (chemo)
- Alectinib vs chemo (stage II–IIIA):
- DFS HR 0.36 (95% CI 0.23–0.56)
- 4-year DFS rate: 74.5% vs 46.3%
- Alectinib vs chemo (ITT; stage IB–IIIA):
- DFS HR 0.35 (95% CI 0.23–0.54)
- 4-year DFS rate: 75.5% vs 47.0%
- CNS DFS: HR 0.37 (95% CI 0.19–0.74)