Robust efficacy findings and unexpectedly low haemorrhagic events were observed with the bispecific antibody but results will only change practice if benefits translate into overall survival and are replicated beyond China
“Squamous non-small cell lung cancer (NSCLC) continues to be associated with a poor prognosis, but the results of the HARMONi-6 trial are noteworthy as they provide evidence that targeting two antigens appears to have advantages over one,” highlights Prof. Myung-Ju Ahn from the Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea, commenting on today’s Presidential Symposium findings (LBA4).
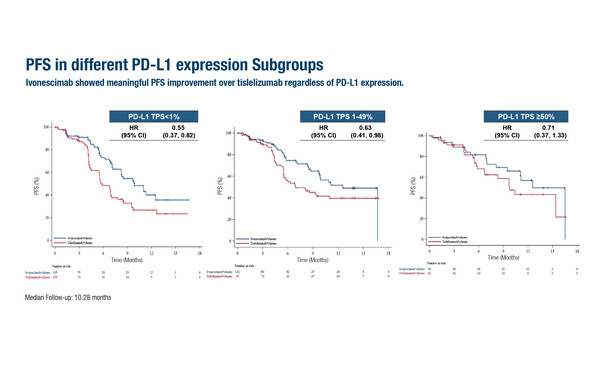
As presented at the ESMO Congress 2025 (Berlin, 17–21 October), in the phase III HARMONi-6 trial, progression-free survival (PFS) was improved by around 4 months when ivonescimab, a bispecific antibody targeting PD-1 and VEGF, was added to chemotherapy compared with tislelizumab plus chemotherapy as first-line treatment for 532 patients with advanced squamous NSCLC. Median PFS – the primary endpoint – was 11.14 months in patients treated with ivonescimab compared with 6.90 months for those receiving tislelizumab (stratified hazard ratio [HR] 0.60; 95% confidence interval [CI] 0.46–0.78; p<0.0001) at a median follow-up of 10.28 months. Consistent benefit was observed across key subgroups. In addition, the HR for median PFS was 0.55 (95% CI 0.37–0.82) in patients with PD-L1 TPS <1% and 0.66 (95% CI 0.46–0.95) in those with PD-L1 TPS ≥1%, which Ahn considers to be, “an insightful and important finding.”
Favourable effects were observed with respect to median duration of response, which was 11.2 months with ivonescimab and 8.4 months with tislelizumab, however, “Results of longer-term follow-up are now needed to determine if PFS effects will translate into significant improvement in overall survival,” notes Ahn.
Safety analyses revealed grade ≥3 treatment-related adverse events (AEs) in 63.9% of patients with ivonescimab and 54.3% with tislelizumab, with a similar proportion of grade ≥3 immune-related AEs reported in each group (9.0% versus 10.2%, respectively). AEs possibly related to VEGF occurred more frequently in the ivonescimab arm, most of which were grade 1–2. Grade ≥3 haemorrhage occurred in 1.9% of patients with ivonescimab and 0.8% with tislelizumab.
Ahn thinks these data are “remarkable” given previous findings with the anti-VEGF agent, bevacizumab, in patients with squamous cell histology: “In a phase II trial 20 years ago, major haemoptysis was observed with bevacizumab in patients with squamous NSCLC (J Clin Oncol. 2004;22:2184–2191), and since then, patients with this histology have generally been excluded from trials with anti-VEGF-1 agents. In HARMONi-6, despite around 30% of patients having haemoptysis history and seemingly being at high risk, the frequency of grade ≥3 haemorrhagic events was surprisingly low.”
The HARMONi-6 trial included only patients from China and therefore data from a global trial in this setting are needed to determine if the findings are more widely applicable. Whether ivonescimab can have a global impact is being assessed by trials currently recruiting in the first-line treatment of squamous and non-squamous NSCLC, including HARMONi-3 trial, comparing ivonescimab plus chemotherapy versus pembrolizumab plus chemotherapy irrespective of PD-L1 expression (NCT05899608), and HARMONi-7, comparing ivonescimab versus pembrolizumab in patients with high PD-L1 expression (NCT06767514). Ongoing trials with other bispecific antibodies will further delineate their role in squamous NSCLC.
Programme details:
Lu S, et al. Phase III study of ivonescimab plus chemotherapy versus tislelizumab plus chemotherapy as first-line treatment for advanced squamous non-small cell lung cancer (HARMONi-6). ESMO Congress 2025 - LBA4