Final OS analysis of the phase III CONTACT-01 trial reports negative results for immunotherapy versus docetaxel in patients progressing on checkpoint inhibitors plus chemotherapy
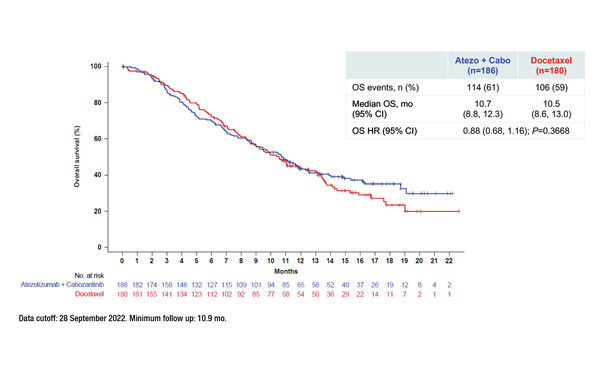
No statistically significant difference in the median overall survival (OS) with atezolizumab plus cabozantinib (10.7 months) versus docetaxel (10.5 months) (stratified hazard ratio [HR] 0.88; 95% confidence interval [CI] 0.68–1.16; p=0.3668) in metastatic non-small cell lung cancer (NSCLC) progressing on anti-PD-L1 inhibitors and chemotherapy was observed in the final OS analysis of the phase III CONTACT-01 trial. Results were presented at the European Lung Cancer Congress 2023 (Copenhagen, 29 March–1 April) (Abstract 6O).
In a group of 366 patients with NSCLC and after a minimum follow-up of 10.9 months, researchers also reported median progression-free survival times of 4.6 months for atezolizumab plus cabozantinib and 4.0 months for docetaxel (stratified HR 0.74; 95% CI 0.59–0.92). Stratification factors were squamous versus non-squamous histology and sequence of prior NSCLC treatment regimens. Objective response rates for atezolizumab plus cabozantinib and docetaxel were 11.8% and 13.3%, respectively, with corresponding median durations of response of 5.6 months and 4.3 months.
Grade 3–4 all-cause adverse events (AEs) occurred at similar rates in the atezolizumab plus cabozantinib (48%) and docetaxel (45%) arms, with discontinuation rates of 17% and 14%, respectively. Grade 5 treatment-related AEs were reported in four patients receiving atezolizumab plus cabozantinib and in one receiving docetaxel.
For Prof. Noemi Reguart from the Hospital Clinic, Barcelona, Spain, the study results are disappointing, but not unexpected. “The CONTACT-01 trial is not the first phase III study that has failed to reach the survival endpoint following encouraging results from an exploratory phase II trial,” she says. The rationale for the CONTACT-01 trial was, in fact, based on the findings of the phase I multitumour COSMIC-021 trial, which demonstrated encouraging clinical activity of cabozantinib plus atezolizumab in patients with advanced NSCLC (J Clin Oncol. 2022;40(Suppl_16):9005–9005). “Targeting angiogenesis has been a focus of research in this context because it is widely known that angiogenesis and immunosuppression are connected processes,” she adds.
Overcoming immune-oncology resistance remains a major challenge in advanced NSCLC, and other approaches are now being explored. “The shift of immune checkpoint inhibitors to first-line therapy has left an unmet need for effective second-line therapies,” she explains. “We must now wait for the results of ongoing phase III trials, such as SAPPHIRE with sitravatinib plus nivolumab, Pragmatica-Lung with ramucirumab plus pembrolizumab, and LEAP-008 with pembrolizumab plus lenvatinib, to establish whether we can expand second-line treatment options for our patients.”
Abstract discussed:
Neal J, et al. CONTACT-01: Efficacy and safety from a Ph 3 study of atezolizumab (atezo) + cabozantinib (cabo) vs docetaxel (doc) monotherapy in patients (pts) with metastatic NSCLC (mNSCLC) previously treated with checkpoint inhibitors and chemotherapy. European Lung Cancer Congress 2023, Abstract 6O
Proffered Paper session 2, 30.03.2023, h. 15:10 – 16:40, Auditorium 1