Results from an interim analysis suggest the antibody–drug conjugate alone or in combination with pertuzumab may have a role in the first-line setting
Trastuzumab deruxtecan (T-DXd) alone and T-DXd plus pertuzumab showed encouraging confirmed overall response rates (cORRs) in the first-line treatment of HER2-positive metastatic breast cancer (mBC), according to an interim analysis of the DESTINY-Breast07 phase Ib/II trial presented at the ESMO Targeted Anticancer Therapies (TAT) Congress 2025 (Paris, 3–5 March) (Abstract 7O).
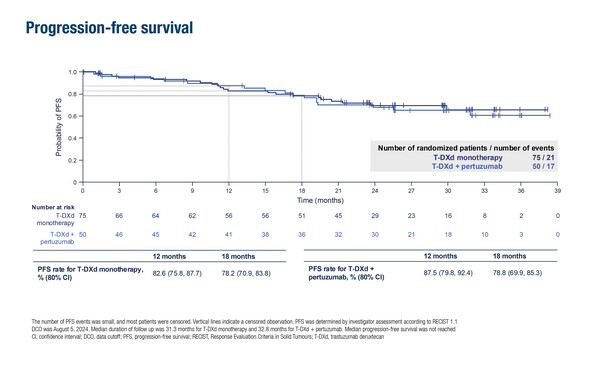
In the study, a total of 75 patients received T-DXd alone and 50 received T-DXd plus pertuzumab, with around 60% having de novo mBC. Included patients had no prior therapy for mBC and a disease-free interval of at least 12 months after any (neo)adjuvant HER2-directed therapy. cORRs were 77.3% in the monotherapy arm and 84.0% in the combination therapy arm. Rates of 18-month progression-free survival (PFS) were 78.2% with monotherapy and 78.8% with the combination. The median duration of response had not been reached yet in both treatment arms.
In an exploratory subgroup analysis in patients with baseline stromal tumour-infiltrating lymphocytes (TILs) ≥20% (n=45) and <20% (n=57), clinical activity was observed with T-DXd monotherapy and T-DXd plus pertuzumab irrespective of baseline stromal TILs values.
“Trastuzumab-based therapy has been a mainstay of systemic therapy for patients with HER2-positive mBC but results with the next-generation antibody–drug conjugate (ADC), T-DXd, suggest further improvements are possible and early in the treatment landscape,” comments Prof. Giuseppe Curigliano from the European Institute of Oncology, Milan, Italy. In a prior study, T-DXd demonstrated a survival benefit over trastuzumab emtansine in patients previously treated with trastuzumab and a taxane (N Engl J Med. 2022;386:1143–1154). “Of course, the encouraging data from this interim analysis should be counterbalanced with the reported toxicity,” he continues. “However, findings in the combination therapy arm may suggest chemotherapy can be omitted when patients are treated with T-DXd plus pertuzumab.”
As reported in Paris, safety data with T-DXd plus pertuzumab were consistent with their individual known profiles. Any grade ≥3 adverse events (AEs) occurred in 53.3% of patients in the monotherapy arm and 60.0% of patients in the combination arm. AEs led to T-DXd discontinuation in 12.0% of patients on monotherapy and 18.0% on combination therapy. Across the two arms, interstitial lung disease/pneumonitis adjudicated as related to study treatment occurred in 14.4% of patients and was of grade 3 severity in one patient.
The number of trials investigating ADC–combination therapies, including with monoclonal antibodies, tyrosine kinase inhibitors and endocrine therapies, has increased considerably over the last few years. According to Curigliano, an important strategy that may further improve on the efficacy of ADCs in breast cancer is their potential synergy with immunotherapy (Clin Med Insights Oncol. 2024;18:11795549241260418) as the findings from the TROPION-Lung04 trial, also presented at ESMO TAT 2025, demonstrated.
“A key pending issue is to assess the most optimal first-line combination in the mBC setting,” he concludes. “We are waiting for the results of the first-line DESTINY-Breast09 trial comparing a taxane, trastuzumab plus pertuzumab to T-DXd alone or in combination with pertuzumab (NCT04784715). This study could be practice changing.” Primary results are expected later this year.
Programme details
André F, et al. Trastuzumab deruxtecan (TDXd) ± pertuzumab (P) in previously untreated HER2+ metastatic breast cancer (mBC): clinical efficacy and exploratory subgroup analyses in DESTINY-Breast07. ESMO Targeted Anticancer Therapies Congress 2025, Abstract 7O
Oral Abstract Session 1, 03.03.2025, h. 15:45 – 17:15, Scene AB