Findings from studies show that patient-centred evidence can inform decisions in early-phase cancer research
The value of using patient-reported outcomes (PROs) in early-phase trials is becoming increasingly recognised, particularly the accurate recording of toxicities that may impact on long-term tolerability for patients, but which are frequently overlooked by clinicians (Oncologist. 2022;27:768–777; ESMO Open. 2024;9:103626).
Studies reported at the ESMO Targeted Anticancer Therapies Asia Congress 2025 (Hong Kong SAR, China, 18–20 July) show that PROs can be a valid instrument to select the target population in clinical trials, but their use needs to be harmonised in early-phase research to get the most out of their potential. In the era of precision medicine, waiting lists for alteration-matched studies are one of the key challenges in early drug development.
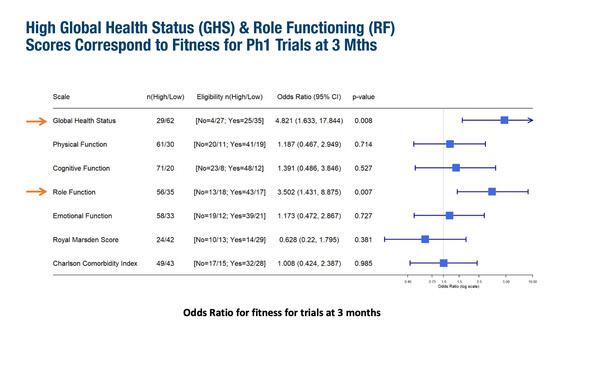
A study from Australia and New Zealand reported that PROs can successfully identify which wait-listed patients will remain suitable for phase I trial inclusion (Abstract 115O). Among 92 eligible patients with solid tumours, fitness for inclusion after 3 months was significantly associated with high baseline values for EORTC QLQ-C30 Global Health Status (odds ratio [OR] 4.82; 95% confidence interval [CI] 1.63–17.84; p=0.008) and Role Functioning (OR 3.50; 95% CI 1.43–8.88; p=0.007). Improved overall survival (OS) was also significantly associated with high versus moderate-low Global Health Status scores (hazard ratio [HR] 2.68; 95% CI 1.3–5.5) and with high versus moderate-low Role Functioning scores (HR 2.06; 95% CI 1.19–3.58). While low baseline Royal Marsden Scores was associated with improved OS, there was no link between Charlson Comorbidity Index and OS.
Professor Boon-Cher Goh from the National University Cancer Institute, Singapore, puts the results into context: “Patients entering phase I trials are a heterogeneous population, with different rates of disease progression that are unknown at screening. These results indicate that PROs could add another layer of understanding, helping to prevent the enrolment of patients who are not sufficiently fit and likely to have a poor outcome to any treatment.”
One of the limitations of the current use of PROs in early studies is the lack of clear objectives (EClinicalMedicine. 2023:64:102228). Findings from OPTIMISE-ROR, reported at the Congress, provide foundational guidance for triallists to systematically integrate PROs into dose-finding oncology trials (Abstract 87O). The project included a methodological review of published trials incorporating PROs, together with a two-round multistakeholder Delphi survey and an independently chaired meeting of 31 international multidisciplinary experts. One of the six recommendations concerns important PROs for assessing the tolerability of investigational treatments: overall side effect impact, symptomatic adverse events and overall health-related quality of life. Another recommendation advocates that these tolerability PROs are assessed across all dose-finding trial settings from dose escalation to expansion and dose optimisation. The guidance also suggests that PRO measurements are considered as part of recommended dose decisions and are used to inform PRO-related endpoints in subsequent phase II trials.
“This project highlights how PROs could be used to their full advantage if a more consistent approach is adopted,” comments Goh, who notes that, if implemented on a broader scale, the consensus-based recommendations are a “very good first step on the way to the development of international guidelines.” But he draws attention to a number of challenges that need to be overcome to enable wider adoption. “Collection of PROs data, including the tools used and the timing and method of their delivery, needs to be standardised. The nature of the data mean that it is subject to a number of influences, including inadequate time given in the clinic for patients to provide comprehensive feedback, and also cultural and age differences in the reporting of side-effects and their impact.” He thinks that technology may help to improve data collection. “Questionnaires, which may be completed while the patient is in the waiting room, could be digitised or even filled in with the aid of a chat bot. In the future, wearables could provide a more complete picture, enabling patients to provide feedback on a daily basis over a longer period of time,” he concludes.
Programme details
Manoharan S, et al. PROPOSE: Predicting phase I trial fitness using patient-reported outcomes and a clinician-completed prognostic index. ESMO Targeted Anticancer Therapies Asia Congress 2025, Abstract 115O
Oral Abstract Session 1, 18.07.2025, h. 15:45 – 17:25, Grand Ballroom 1
Alger E, et al. International consensus-driven recommendations for patient-reported outcome research objectives in early phase dose-finding oncology clinical trials: OPTIMISE-ROR. ESMO Targeted Anticancer Therapies Asia Congress 2025, Abstract 87O
Oral Abstract Session 1, 18.07.2025, h. 15:45 – 17:25, Grand Ballroom 1