In the TROPION-Lung04 study, no new safety signals were observed with datopotamab deruxtecan plus durvalumab in patients with untreated advanced disease
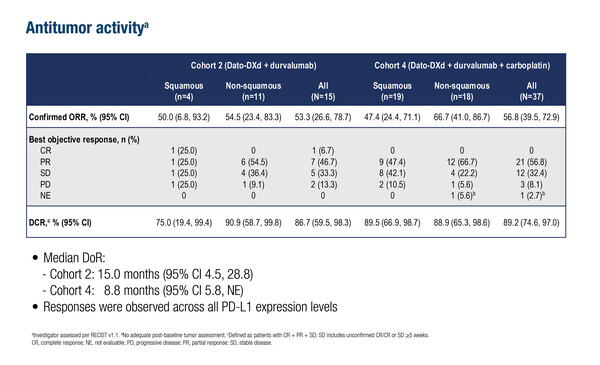
As presented at the ESMO Targeted Anticancer Therapies (TAT) Congress 2025 (Paris, 3–5 March), the combination of the TROP-2-directed antibody–drug conjugate (ADC) datopotamab deruxtecan (Dato-DXd) with durvalumab or with durvalumab plus carboplatin led to confirmed objective response rates of 53.3% and 56.8%, respectively, in the TROPION-Lung04 phase Ib trial (Abstract 8O). The results are from an updated analysis of two cohorts of treatment-naïve patients with advanced non-small cell lung cancer (NSCLC) without actionable genomic alterations who received Dato-DXd plus durvalumab either without carboplatin (cohort 2; n=15) or with carboplatin (cohort 4; n=37).
Disease control rates were more than 85% in both cohorts and responses were observed in squamous and non-squamous histologies, and across PD-L1 expression levels. Median progression-free survival (PFS) was 7.3 months (95% confidence interval [CI] 2.0–29.5 months) in cohort 2 and 8.7 months (95% CI 5.6–10.1 months) in cohort 4.
In the primary endpoint analysis, safety profiles were consistent with those expected from the individual agents. Grade ≥3 treatment-emergent adverse events occurred in 60.0% of patients in cohort 2 and 70.3% in cohort 4, most commonly anaemia and neutropenia in cohort 4 (both 24%). There was one case in each cohort of grade ≥3 adjudicated treatment-related interstitial lung disease/pneumonitis.
While immune checkpoint inhibitors have improved outcomes for untreated patients with NSCLC without actionable genomic alterations, most patients eventually experience disease progression (Ther Adv Med Oncol. 2020:12:1758835920937902). Building on the efficacy seen with Dato-DXd alone compared with chemotherapy in pre-treated patients in the TROPION-Lung01 phase III study (J Clin Oncol. 2025;43:260–272), a subgroup analysis of the TROPION-Lung02 phase I trial showed signs of antitumour activity with Dato-DXd in combination with pembrolizumab in treatment-naïve patients (J Clin Oncol. 2024;42(16_suppl):8617). The benefits of an ADC alone and as combination therapy have also been demonstrated in the first-line setting in the DESTINY-Breast07 phase Ib/II trial presented at ESMO TAT 2025.
Whether early-phase efficacy and safety with ADC–immunotherapy combinations will translate into positive phase III findings in the first-line NSCLC setting is now being tested in several trials including TROPION-Lung07 (NCT05555732), TROPION-Lung08 (NCT05215340), AVANZAR (NCT05687266) and EVOKE-03 (NCT05609968).
Programme details
Cuppens K, et al. First-line (1L) datopotamab deruxtecan (Dato-DXd) + durvalumab ± carboplatin in advanced or metastatic non-small cell lung cancer (a/mNSCLC): Results from TROPION-Lung04 (cohorts 2 and 4). ESMO Targeted Anticancer Therapies Congress 2025, Abstract 8O
Oral Abstract Session 1, 03.03.2025, h. 15:45 – 17:15, Scene AB