Studies investigate the discriminatory capacity of existing prognostic scales and the factors associated with survival to help select patients who may benefit from participation in early phase trials
Selecting the right patients for a phase I trial is still challenging and there is a need for greater understanding of prognostic variables for long-term survival as two retrospective single-centre cohort studies presented at the ESMO Targeted Anticancer Therapies Congress 2024 (Paris, 26–28 February) highlight.
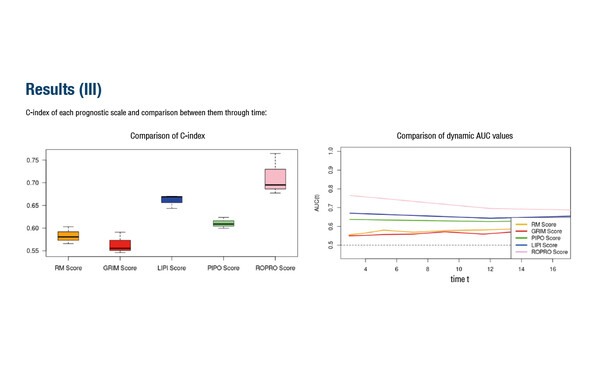
In a first study, five prognostic scoring tools commonly used in oncology were compared among 844 patients treated mainly with immune checkpoint inhibitors from 2016 to 2023 (Abstract 28O). The highest dynamic area under the curve (AUC) values were reported with the Real wOrld PROgnostic (ROPRO) scale and Lung Immune Prognostic Index (AUC of 0.76 and 0.67, respectively), with correspondingly greater concordance index (C-index) scores, while the lowest performance was seen with the PIPO web tool (0.63), the Gustave Roussy Immune (0.56) and Royal Marsden Hospital (0.56) scales. Receiving molecularly matched treatment or checkpoint inhibitors and having high levels of albumin but low levels of leukocytes and neutrophils were linked to long- versus short-term survival (OS >3 years versus <3 years, respectively) in a second study (Abstract 29O).
Over the last few decades, phase I trials have evolved from having mainly a safety perspective to also providing preliminary evidence of efficacy. “Contemporary trials aim to rapidly identify signs of activity in the whole patient population or in selected subgroups, using a more precise approach than a decade ago. Knowing that patients may respond to treatment brings even more responsibility to ensure that the most appropriate patients are selected, while still balancing the risks,” says Prof. Fabrice Barlesi from Institut Gustave Roussy, Villejuif, France.
Typical trial eligibility entry criteria include an anticipated life expectancy of at least 3 months; however, up to 20% of patients do not survive the first 90 days of phase I trial entry (BMC Cancer. 2011;11:426). Accurate prognostic tools help clinicians with the often-difficult decision-making process about trial suitability and to engage in discussions with patients, managing their expectations. “Currently, many prognostic scales are available but these may not yet be globally applicable, with results dependent on the tumour type and stage,” continues Barlesi. “It is important that prognostic tools are easy for the clinician to apply but also sophisticated enough that they take into account the effects of the wide range of new treatment modalities, from antibody–drug conjugates to immunotherapies, that the patient may have received already or will receive in the trial.”
Barlesi concludes, “Further improvements of current selection strategies may be derived from modelling and artificial intelligence, interrogating large databases from multiple centres and countries, to help us gain a better understanding of the factors that most influence survival and suitability for today’s modern trial designs and novel treatments.”
Abstracts dicussed:
Sangro P, et al. Comparison of prognostic scores in oncology patients participating in phase 1 clinical trials with immunotherapy. ESMO Targeted Anticancer Therapies Congress 2024, Abstract 28O
Abstract Session 2, 27.02.2024, h. 15:45 – 17:15, Amphitheatre Bordeaux
Mirallas O, et al. Clinical characterization of long-term survivors in phase I clinical trials. ESMO Targeted Anticancer Therapies Congress 2024, Abstract 29O
Abstract Session 2, 27.02.2024, h. 15:45 – 17:15, Amphitheatre Bordeaux