Strategies to target MTAP deletion, KRAS G12D and ESR1 mutations, and CDK4 and CDK2 further expand the landscape of precision oncology
Despite a growing number of targeted therapies being approved in the last ten years, not all tumours present actionable alterations and much of the success of precision oncology depends on the identification of new druggable targets that may ultimately drive drug development. At the ESMO Congress 2024 (Barcelona, 13–17 September), some early-phase trials described promising strategies to target emerging actionable mutations in different solid tumours, such as methylthioadenosine phosphorylase (MTAP) deletion, KRAS G12D mutation, and CDK4 and 2, reporting good tolerability and antitumour activity for novel targeted agents.
In a first phase I trial, the protein arginine methyltransferase 5 (PRMT5) inhibitor AMG193 – designed to selectively induce synthetic lethality in tumours with MTAP deletion while sparing normal cells – demonstrated a favourable safety profile with no evidence of clinically significant myelosuppression in 167 patients with advanced MTAP-deleted solid tumours (Abstract 604O). Approximately 15% of cancers exhibit a loss of MTAP gene, which is located adjacent to the cyclin-dependent kinase inhibitor 2A (CDKN2A) gene and the interferon gene cluster (Front Oncol. 2023;13:1264785).
Treatment with AMG193 led to objective response rates (ORRs) of 11.8%, 8.7% and 10.5% in patients with non-small cell lung cancer (NSCLC), pancreatic ductal adenocarcinoma and biliary tract cancer, respectively. Commenting on these findings, Dr Lillian Siu from the Princess Margaret Cancer Centre, Toronto, Canada says, “The results from this study are exciting and provide proof-of-concept that this agent can induce synthetic lethality in MTAP-deleted tumours. Moving forward, it will be interesting to explore the rational combination of AMG193 with other agents and also to investigate the potential benefits of using AMG193 in earlier-stage cancers before resistance to this pathway develops.”
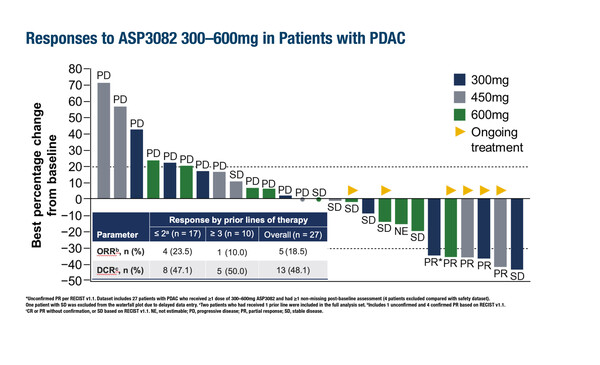
A second phase I dose-escalation study investigated the clinical activity of the first-in-class KRAS G12D selective protein degrader ASP3082 in 111 patients with advanced pancreatic cancer, colorectal cancer and NSCLC and was shown to have an acceptable safety profile (Abstract 608O). The KRAS G12D mutation is present in more than one in three pancreatic cancers, about one in eight colorectal cancers, and in several other cancer types (Cancer Discov. 2022;12:924–937). At the dose of ASP3082 300–600 mg intravenously once weekly (n=13), the ORR was 23.1% and the disease control rate (DCR) was 84.6% in NSCLC and 18.5% and 48.1%, respectively, in pancreatic ductal adenocarcinoma. “Targeting the KRAS G12D mutation is generating tremendous interest as it occurs in several tumour types at high rates. The trial results are especially notable for those difficult-to-treat cancers such as pancreatic cancer for which encouraging responses were reported,” notes Siu.
Finally, two early-phase trials provide further insights on how to refine treatment strategies targeting CDK2. Activation of CDK4/6 is implicated in breast, ovarian and non-small cell lung cancers, among others (FASEB J. 2024;38:e23734). Co-targeting CDK2 and CDK4/6 can overcome resistance to aromatase and CDK4/6 inhibitors in hormone receptor positive breast cancers (NPJ Precis Oncol. 2022;6:68). A phase Ib/II study in patients with metastatic breast cancer (mBC, n=26) and other solid tumours (n=7) demonstrated that a combination of selective inhibitors of CDK4 (PF-07220060) and CDK2 (PF-07104091) was generally well-tolerated with promising antitumour activity (Abstract 618MO). For patients with mBC, median progression-free survival was 8.3 months and the clinical benefit response rate was 50.0%. Of the patients with mBC and measurable disease (n=18), 5 had partial responses (all with known ESR1 mutations) and 5 had stable disease. Commenting on the results, Siu says, “This is a clinically relevant strategy as all patients with mBC in the study had previously received CDK4/6 inhibitors. Resistance to these agents occurs and such patients will require new treatments. It is also noteworthy that patients with ESR1 mutations appear to be particularly responsive to the combination used in this study and may serve as a selective biomarker for future evaluations.”
Selective CDK2 inhibition was also examined in a phase I trial of INCB123667 in patients with advanced-stage solid tumours (Abstract 617MO). Among 84 patients in this dose escalation study, INCB123667 was generally well tolerated, with only 4 patients discontinuing because of an adverse event. Of the 76 efficacy-evaluable patients, 8 had partial responses (ovarian, endometrial and triple-negative breast cancers) and 40 had stable disease. Of the 68 patients with ovarian cancer participating in dose escalation and expansion, 2 had a complete response, 12 had a partial response and 38 had stable disease; the majority of these patients had cyclin E1 overexpression and slightly less than half had CCNE1 amplification. “It is great to see bona fide monotherapy tumour response in this study,” says Siu. “Looking to the future, it will be important to determine how best to enrich and select patient populations for treatment with this agent.”
Siu concludes, “Advancing novel targeted agents to the clinic will require a greater understanding of how to combine them rationally with other treatments, with a focus on synergistic rather than additive effects, and taking the therapeutic index into consideration. It will also be critical to examine their use in earlier-stage cancers, including in the contexts of peri-operative treatment and minimal residual disease.”
Programme details
Simonelli M, et al. Safety and tolerability of INCB123667, a selective CDK2 inhibitor, in patients (Pts) with advanced solid tumors: A phase I Study. ESMO Congress 2024, Abstract 617MO
Mini Oral Session – Developmental therapeutics, 14.09.2024, h. 14:45 – 16:15, Oviedo Auditorium – Hall 3
Yap TA, et al. Phase Ib/II first-in-class novel combination trial of next generation CDK4-selective inhibitor PF-07220060 and next generation CDK2-selective inhibitor PF-07104091 in HR+ HER2- metastatic breast cancer and advanced solid tumors. ESMO Congress 2024, Abstract 618MO
Mini Oral Session – Developmental therapeutics, 14.09.2024, h. 14:45 – 16:15, Oviedo Auditorium – Hall 3
Park W, et al. Preliminary safety and clinical activity of ASP3082, a first-in-class, KRAS G12D selective protein degrader in adults with advanced pancreatic (PC), colorectal (CRC), and non-small cell lung cancer (NSCLC). ESMO Congress 2024, Abstract 608O
Proffered Paper Session 2 – Developmental therapeutics, 15.09.2024, h. 14:45 – 16:15, Salamanca Auditorium – Hall 5
Sacher AG, et al. Phase I dose escalation and initial dose expansion results of AMG 193: A MTA-cooperative PRMT5 inhibitor, in patients (pts) with MTAP-deleted solid tumors. ESMO Congress 2024, Abstract 604O
Presidential Symposium III – Eyes to the future, 16.09.2024, h. 16:30 – 18:15, Barcelona Auditorium – Hall 2