Agents targeting B7-H3 and CEACAM6 pass their initial clinical tests, according to results from first-in-human trials
Two studies presented at the ESMO Asia Congress 2024 (Singapore, 6–8 December) highlight promising new immune-related targets. In the first study, DB-1311/BNT324, a B7-H3-targeting antibody linked to a DNA topoisomerase I inhibitor, demonstrated low rates of grade ≥3 adverse events (AEs) and promising antitumor activity in patients with pre-treated advanced/metastatic solid tumours unselected for B7-H3 expression (Abstract 57O). Overexpression of B7-H3, an immune checkpoint protein, has previously been shown to correlate with tumour growth, invasion, metastasis, malignant stage and recurrence rate (Cancer Chemother Pharmacol. 2018;81:245–253).
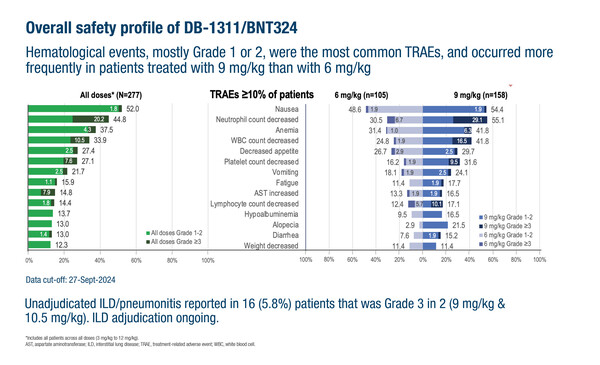
In the phase I/II trial, DB-1311/BNT324 was tested at 3–12 mg/kg IV Q3W, with 9 mg/kg established as the maximum tolerated dose. Grade ≥3 treatment-related AEs occurred in 23.8% of patients with 6 mg/kg and 51.9% with 9 mg/kg, which were most often haematological.
DB-1311/BNT324 demonstrated encouraging tumour responses at 6 mg/kg and 9 mg/kg, particularly in 67 patients with small cell lung cancer (SCLC), where unconfirmed objective response rates (uORR) were 54.5% and 58.8%, respectively. Early signs of activity were also seen in non-small cell lung cancer (uORR of 22.0%) and castration-resistant prostate cancer (uORR of 28.0%).
Commenting on these findings, Dr Daniel Tan from the National Cancer Centre Singapore says, “The early uORR results are promising, especially in difficult-to-treat SCLC. These data indicate that B7-H3, which is from the same family as PD-L1, is a target worth developing further in this novel antibody–drug conjugate format.”
A second phase Ia dose-escalation study assessed DNP002, a monoclonal antibody that binds to carcinoembryonic antigen-related cell adhesion molecule (CEACAM) family proteins, particularly CEACAM6, which is differentially expressed in various cancers and is associated with poorer overall survival (Discov Oncol. 2024;15:186) (Abstract 60O). To date, 16 Korean patients with advanced solid tumours have received DNP002, with two dose-limiting toxicities at 0.25 mg/kg and dose-dependent neutropenia observed. Among 12 evaluable patients, there was 1 partial response, which lasted 30 weeks and was seen in a patient with oesophageal cancer who received 18 doses of DNP002 (0.03 mg/kg) and experienced a 69% reduction in tumour burden. Tumour reduction effects were also observed in 1 patient with neuroendocrine carcinoma and 1 patient with a myxoid neoplasm. Six patients had stable disease. Significant increases in inflammatory cytokines and cytotoxic T-cells expressing CD69 were noted with DNP002 treatment.
Tan comments, “Previous attempts to target CEACAM have largely centred around CEACAM5, but there is growing interest in CEACAM6, which appears to play a complex role within the tumour microenvironment. The data presented provide evidence that CEACAM6 is a clinically valid target, and again highlight the diverse mode of action with therapeutic antibodies – underscoring the importance of pharmacodynamic studies to identify rational combinations.”
With so many clinical unmet needs remaining, Tan thinks that the search for new targets and agents with novel mechanisms of action is as urgent as ever. He concludes, “DB-1311/BNT324 originated from China and DNP002 is being developed in the Republic of Korea. The promising findings with these agents showcase the huge growth in biotech companies and innovation ongoing in the Asian region.”
Programme details
Cheng Y, et al. A phase I/II multicenter, first-in-human study of DB-1311/BNT324 (a novel B7H3 ADC) in patients with advanced solid tumors. ESMO Asia Congress 2024, Abstract 57O
Proffered Paper Session: Developmental and Precision Medicine, 06.12.2024, h. 10:15 – 11:45, Hall 402
Choi W, et al. A phase Ia study of DNP002, a monoclonal antibody that binds to tumor-associated CEACAM family proteins, in patients with advanced solid tumors: Interim results from dose escalation study. ESMO Asia Congress 2024, Abstract 60O
Mixed Session – Proffered Paper and Mini Oral: Developmental and Precision Medicine, 06.12.2024, h. 16:45 – 18:20, Hall 406