Data from studies presented highlight the importance of integrating and incorporating comprehensive genomic profiling into clinical practice across a broad range of tumour types
At the ESMO Congress 2024 (Barcelona, 13–17 September), data from several Drug Rediscovery Protocol-like Clinical Trials (DLCTs) provided evidence for the feasibility, early efficacy and safety achievable with personalised treatments based on comprehensive genomic profiling (CGP).
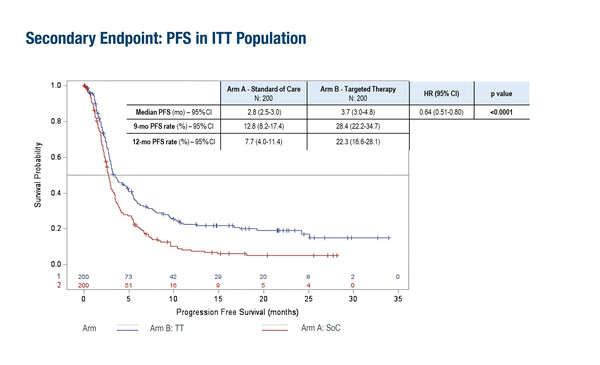
The potential of CGP and molecular tumour board discussions to expand precision cancer medicine was highlighted in the ROME trial, a randomized multi-basket phase II study to be presented in a Presidential Symposium (LBA7). In this study, 400 pre-treated patients with advanced or metastatic solid tumours (covering 38 different histologies) and bearing actionable mutations, reported a significantly improved objective response rate (ORR; 17.0% versus 9.5%, p=0.027) and median progression-free survival (PFS; 3.7 months versus 2.8 months, p<0.0001) and no increase in toxicity with targeted therapy compared with standard-of-care (SoC) treatment. The benefits of targeted therapy were particularly evident in an exploratory analysis of patients with high tumour mutational burden or microsatellite stable tumours who received immunotherapy (12-month PFS: 32.7% versus 6.3%, respectively; p=0.001).
The value of molecular profiling to inform precision oncology was also highlighted in results presented from FINPROVE, a prospective, open label randomised combined basket and umbrella DLCT in which 130 patients were treated in multiple cohorts based on the molecular profile of their tumour (Abstract 81P). Treatment recommendations were guided by a molecular tumour board based on DNA profiling using the ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT) criteria (Ann Oncol. 2018;29:1895–1902). Patients with combined ESCAT tiers I and II experienced the longest PFS (137 days) compared with other tiers.
The importance of CGP to identify actionable mutations and guide targeted therapy decisions was corroborated in results describing CGP profiling from a pooled analysis of 144 real-world studies (Abstract 121P). Actionable genomic mutations were reported in 59.8% of patients tested, of whom 15.6% received targeted therapy. The ORR was 23.9%, and CGP-matched treatment decisions appeared to correlate with improved survival compared with conventional therapy.
“DLCTs explore the effects of biomarker-driven treatments outside their approved indication(s) across a broad range of tumour types, thus enabling the study of personalised therapy in large numbers of patients,” says Dr Vivek Subbiah from the Sarah Cannon Research Institute, Nashville, TN, USA, commenting on the studies’ findings. “The data presented highlight the importance of integrating and incorporating CGP into clinical practice and may impact treatment guidelines leading to a shift towards personalised treatment plans, with incorporation of genomic data into the decision-making process. However, changes in resource allocation and the infrastructure of clinics are needed to provide investment in genomic testing and train healthcare teams to interpret and act on genomic data.” According to Subbiah, DLCTs facilitate data sharing and the promotion of innovative study designs, while the collaborative nature of DLCTs accelerate the pace of research, scalability and evidence generation. The benefits of collaboration and merging data between multiple DLCTs were reviewed in a presentation on PRIME-ROSE, a European Cancer Mission project on precision medicine trials across a broad variety of tumour types (Abstract 197P). The project involves 11 ongoing or planned DLCTs that are aligned in terms of their protocols and endpoints, so that cohorts from different trials may be merged, thus improving recruitment and evidence generation.
Subbiah suggests that the data from DLCTs may be required for cost–benefit assessments for new therapies, while regulatory bodies may develop a more flexible and adaptive framework to assist in approving therapies based on real-world evidence and genomic data. Review processes could potentially be expedited for treatments showing significant promise in DLCTs, with continuous monitoring to ensure long-term safety and efficacy. However, DLCTs have some limitations. “The lack of generalisability to all patient populations and the absence of control arms can be an issue. In addition, interpretation of the complex genomic datasets requires specialised expertise and infrastructure, while ethical concerns may arise relating to equity of patient access to, and receipt of, these personalised treatments. However, their benefits seem to outweigh any potential limitations,” he concludes.
Programme details
Kringelbach TM, et al. PRIME-ROSE: Merging clinical outcome data from DRUP-like clinical trials. ESMO Congress 2024, Abstract 197P
Poster Display – Biomarkers & translational research, 15.09.2024, h. 12:00 – 13:00, Hall 6
Jalkanen K, et al. FINPROVE – The Finnish National Study to Facilitate Patient Access to Targeted Anti-cancer Drugs – preliminary data after two years of enrollment. ESMO Congress 2024, Abstract 81P
Poster Display – Biomarkers & translational research, 15.09.2024, h. 12:00 – 13:00, Hall 6
Zerdes I, et al. Comprehensive cancer genome profiling as supportive tool for treatment decision-making in patients with metastatic solid tumors: real-world evidence-based meta-analysis and nationwide cancer registry data implementation. ESMO Congress 2024, Abstract 121P
Poster Display – Biomarkers & translational research, 15.09.2024, h. 12:00 – 13:00, Poster Hall 6
Botticelli A, et al. The Rome trial from histology to target: The road to personalize targeted therapy and immunotherapy. ESMO Congress 2024, LBA7
Presidential Symposium III – Eyes to the future, 16.09.2024, h. 16:30 – 18:15, Barcelona Auditorium – Hall 2