Results of extensive genomic profiling and a Time-to-Event Continual Reassessment Method show some promise in investigating new treatments and targets in early-phase trials, despite not being ready for routine research
Faster, more streamlined ways of investigating the value of new treatments and targets in early-phase trials are urgently needed in oncology as the number of distinct tumour molecular profiles identified is increasing. Novel tools and methods are discussed at the ESMO Targeted Anticancer Therapies Congress 2023 (Paris, 6–8 March) providing some insights into progress in the field.
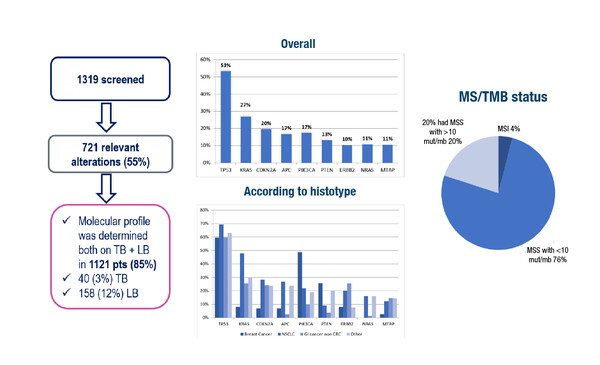
In the first study, the phase II ROME trial, the results of extensive genomic profiling (EGP) of progressive, metastatic tumours were used to randomise patients to either a relevant targeted treatment or standard of care for that tumour type (NCT04591431). Tissue (collected <6 months) and blood samples were analyzed with a centralized NGS assay. According to 2-year results presented at the Congress, EGP with molecular tumour board discussions modified the treatment strategy in around one-third of the patients (Abstract 70MO). Among 1,319 patients with breast, gastrointestinal (GI) non-colorectal, lung or other cancers, the majority of whom (85%) had EGP performed on tissue and liquid biopsy, the most commonly mutated gene was TP53 (53%), followed by KRAS (27%), CDKN2A/B (20%), APC (17%), PIK3CA (17%), PTEN (13%), ERBB2 (10%), NRAS (11%) and MTAP (11%). Tumours in 4% of patients had microsatellite instability, while 76% and 20% had microsatellite stability with <10 mutations/Mb and >10 mutations/Mb, respectively.
Among the patients assigned to treatment based on the EGP and molecular tumour board discussions, 316 patients (24%) with actionable mutations were randomised to treatment with a relevant targeted agent or to standard of care. While 167 patients had no actionable genetic alterations, molecular tumour board discussions led to referral to another trial (n=58), modification of standard treatment (n=28) or, in the case of potential germline alterations, genetic counselling (n=81).
Although the results suggest that EGP could be useful in assigning patients to appropriate targeted treatment, Dr Stefanie Hayoz, head of statistics of the Swiss Group for Clinical Cancer Research (SAKK), Bern, Switzerland, highlights that the value of treatment assignment in terms of patient outcomes has yet to be confirmed. “We do not know from these data whether the EGP and molecular tumour board discussion were really useful in the sense that the patients profited from the therapy in terms of a better outcome,” she notes. “Regarding the methodology, extensive profiling is in some respects a double-edged sword: a large number of different mutations were revealed, many of which will be infrequent, and while it increases opportunities for effective targeted treatment, it raises challenges for clinical trials.” She continues, “It is not feasible, or economically viable, to run trials for patients with each rare tumour mutation, nor is it possible to arrange adequately powered subgroup analyses. Instead, we urgently need novel approaches that are able to provide meaningful results for what are individually small groups of patients but together make up a large population of patients.” Also, widespread use of extensive profiling currently faces a number of hurdles, not least of which is a lack of access to the technology, particularly in centres and countries with limited resources. Use of a central location to conduct profiling may be a potential solution, says Hayoz, although any resulting treatment delay would need to be taken into account.
A second presentation at the Congress described the successful modification of a Time-to-Event Continual Reassessment Method (TiTE-CRM) model to include consolidation immunotherapy in the arm of the phase I CONCORDE platform trial looking at the use of an ATR inhibitor with or without consolidation durvalumab following radiotherapy (Abstract 28MO). “The TiTE-CRM model is a time-efficient way of determining maximum tolerated doses in such trials when long-term toxicities are anticipated. However, these models can be difficult to implement and rely on a lot of assumptions,” says Hayoz, commenting on the study where the investigators simulated and evaluated two populations across various scenarios with different toxicity profiles and combined in the same adapted TiTE-CRM model. The model was found to answer the research question under a wide range of scenarios, although performance decreased when the observed toxicity in participants receiving durvalumab consolidation treatment differed from original assumptions.
“This is a very interesting methodology, and it is commendable that such methods are being investigated and presented. When we have the full findings, it will serve as a good template for other clinical trial researchers wishing to conduct similar trials,” observes Hayoz. “The fact that the investigators simulated several scenarios to assess the robustness of what is quite a complex model design is very important. Although the finding that the model performance decreases when the assumed and observed toxicities differ is to be expected, it is an important point to make. Any model, however elaborate, is only as good as its assumptions.”
Abstracts discussed:
Botticelli A, et al. Genomic profiling to expand precision cancer medicine in the real world: The ROME trial. ESMO Targeted Anticancer Therapies Congress 2023, Abstract 70MO
Mini Oral Session: Optimal tools and methods for developing and implementing precision therapeutics, 06.03.2023, h. 11:45 – 12:45, Amphitheatre Bordeaux
Kendall J, et al. Adapting the Time-to-Event Continual Reassessment Method (TiTE-CRM) to include consolidation immunotherapy in a phase I drug-radiotherapy platform trial. ESMO Targeted Anticancer Therapies Congress 2023, Abstract 28MO
Mini Oral Session: Optimal tools and methods for developing and implementing precision therapeutics, 06.03.2023, h. 11:45 – 12:45, Amphitheatre Bordeaux