Safety and biomarker status data of the PARP7 inhibitor RBN-2397 and the IgG4 monoclonal antibody SRK-181 presented at the ESMO TAT Congress 2023
Two phase I trials presented at the ESMO Targeted Anticancer Therapies Congress 2023 (Paris, 6–8 March) provide insights into possible new treatment approaches urgently needed for patients who either do not respond or become resistant to immunotherapy over time.
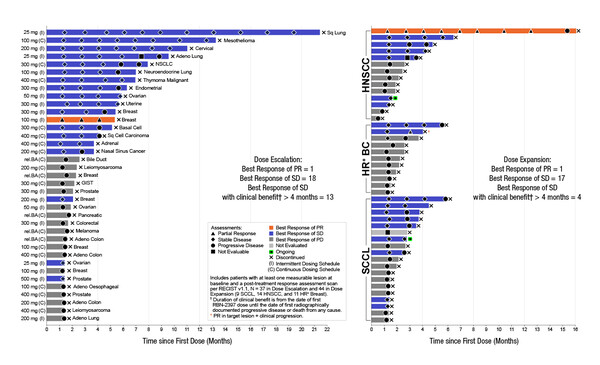
The first study reported results of treatment with the first-in-class PARP7 inhibitor, RBN-2397, which has beneficial effects on type I interferon signalling and the adaptive immune response (Abstract 27O). Among heavily pretreated patients receiving the recommended phase II dose in expansion cohorts, RBN-2397 was associated with stable disease (SD) for 3+ months in 5/19 patients with advanced squamous cell carcinoma (SCC) of the lung and 2/11 patients with hormone receptor-positive breast cancer. In the head and neck SCC expansion cohort, 1/14 patients achieved a PR for 12+ months and 4/14 achieved SD for 4+ months.
Among all 103 patients treated in the dose-escalation and expansion phases, treatment-related adverse events (TRAEs) comprised dysgeusia (37.9%), fatigue (20.4%), nausea (17.5%) and decreased appetite (13.6%), and grade 3–4 events included aspartate aminotransferase increase (4.9%), alanine aminotransferase increase (3.9%) and anaemia (2.9%). Baseline biopsies showed PARP7 expression to be consistently higher in tumour cells compared with stromal cells. In on-treatment biopsies, there was evidence of increases in CD8+ T cells and/or granzyme B expression in most patients across tumour types, confirming RBN-2397-associated induction of adaptive immunity. Investigation of RBN-2397 in combination with pembrolizumab is ongoing in patients with lung SCC (NCT05127590).
“Targeting PARP7, which has been found to be expressed in several tumour types, is a very interesting approach,” says Prof. Alessandra Curioni-Fontecedro from HFR Fribourg – Kantonsspital, Switzerland. “The reasonable number of patients enrolled in the trial enables us to get some good insights into the activity of RBN-2397. Administered as a single agent, RBN-2397 seems to be quite tolerable, despite side-effects that can be related to the class of drug. And the number of patients achieving stable disease was quite high,” she notes. “It will be very interesting to see more information on the tumour expression status of PARP7, to gain more understanding of any association between expression, and the degree of expression, with patient outcomes.”
Promising results were also presented at the Congress from the ongoing DRAGON trial investigating SRK-181, an IgG4 monoclonal antibody that selectively binds to latent transforming growth factor-beta 1 (TGFβ1), which is associated with primary resistance to PD-(L)1 blockade (Abstract 1O). So far, no dose-limiting toxicities have been observed. During dose-escalation of SRK-181 monotherapy in 19 patients who had received a median of four prior lines of therapy, the most common TRAEs were fatigue (n=3), decreased appetite (n=2) and nausea (n=2). Stable disease was achieved by 8 patients and was prolonged (25–42 weeks) in 3 patients with ovarian cancer. Among 15 patients with PD-(L)1-resistant disease receiving SRK-181 plus anti-PD-(L)1 treatment, the most common TRAEs were maculo-papular rash (n=5), pruritus (n=4), rash (n=3), diarrhoea (n=2) and pemphigoid (n=2). One patient with anti-PD-1-resistant clear cell renal cell carcinoma (ccRCC), who stayed on study for 30 weeks, achieved a confirmed partial response (PR). Stable disease was observed in 9 patients and was prolonged (>16 weeks) in 5. In an ongoing expansion cohort of patients with PD-(L)1-resistant tumours, two confirmed PRs have been observed in patients with ccRCC receiving SRK-181 plus anti-PD-(L)1 therapy. Target engagement of SRK-181 was indicated by an increase in the levels of circulating TGFβ1 following treatment.
“TGFβ blockade has a strong preclinical rationale and has been investigated in the clinical setting with variable results (EBioMedicine. 2022;86:104380),” says Curioni-Fontecedro. “However, although this is not a new approach, the specificity of SRK-181 points to an improved non-clinical toxicity profile compared with previously investigated broad-spectrum TGFβ inhibitors (Int J Toxicol. 2021;40:226–241). Indeed, the safety data from the DRAGON trial look promising. The trial included only a very small number of patients and so we must consider the data preliminary. That said, the signals of efficacy are encouraging, particularly bearing in mind that the patients were heavily pretreated,” she notes.
Results from the two studies presented highlight the need to identify novel immunotherapy targets to increase the number of therapeutic options for patients.
Curioni-Fontecedro concludes: “These new agents may play an important role in helping to develop combinatorial treatments for patients with resistance to existing immunotherapies, particularly in the case of RBN-2397, the immunomodulatory effects of which provide a rationale for its use in combination with immunotherapy.”
Abstract discussed:
Yap TA, et al. First-in-class first-in-human phase I trial of RBN-2397 in patients with advanced solid tumors validates PARP7 as a novel anticancer therapeutic target. ESMO Targeted Anticancer Therapies Congress 2023, Abstract 27O
Proffered Paper Session, 07.03.2023, h. 17:05 – 18:20, Amphitheatre Bordeaux
Yap TA, et al. Safety, pharmacokinetics, efficacy, and biomarker results of SRK-181 (a latent TGFβ1 inhibitor) from a phase I trial (DRAGON trial). ESMO Targeted Anticancer Therapies Congress 2023, Abstract 1O
Proffered Paper Session, 07.03.2023, h. 17:05 – 18:20, Amphitheatre Bordeaux