Newly presented phase III trial and real-world findings reassure on the use of the gamma secretase inhibitor
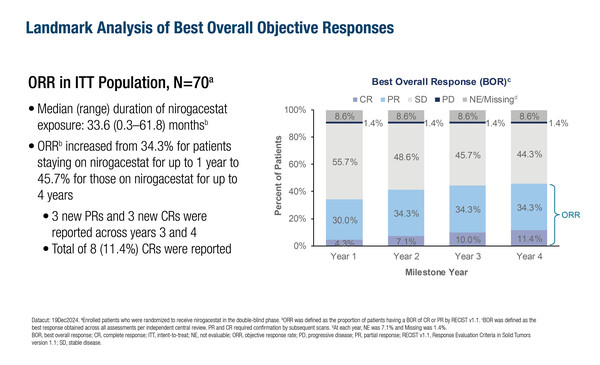
In the phase III DeFi trial, longer-term treatment with the oral gamma secretase inhibitor nirogacestat increased the objective response rate from 34.3% (for up to 1 year of treatment) to 45.7% (for up to 4 years of treatment) in patients with progressing desmoid tumours (Abstract 67MO). Updated data were presented at the ESMO Sarcoma and Rare Cancers Congress 2025 (Lugano, 20–22 March).
Nirogacestat was approved in the USA for the treatment of adult patients with progressing desmoid tumours who require systemic treatment following positive findings from the DeFi trial first reported at the ESMO Congress 2022 and published in March 2023 (N Engl J Med. 2023;388:898–912). The updated analysis included an additional 13 months of follow-up from the previous report, with median exposure to nirogacestat of 33.6 months (range, 0.3–61.8 months). Across years 3 and 4, there were three additional complete responses and three additional partial responses.
The incidence and severity of treatment-emergent adverse events (TEAEs) decreased after year 1. There were five TEAE-related dose reductions after the primary analysis, all of which occurred between years 2 and 3. Patient-reported outcomes (PROs) showed improvements in pain, desmoid tumour-specific symptom burden, physical and role functioning, and general quality of life that were sustained for up to 4 years of treatment.
“It is reassuring that no new adverse effects were observed with prolonged use of nirogacestat and that the incidence and severity of TEAEs declined with time,” comments Dr Antonia Digklia from the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland. “However, it would be interesting to see more long-term data on the incidence of non-melanoma skin cancers, which have previously been reported with nirogacestat. For now, all patients receiving this treatment should have regular dermatologic evaluation.”
Data from DeFi were broadly confirmed by real-world findings from the French Sarcoma Group’s DeNi study (Abstract 83P). Among 55 previously treated patients with desmoid tumours in this compassionate use programme, 52% achieved partial responses and 39% had stable disease after a median follow-up of 9.6 months. Thirty-eight patients (73%) reported pain reduction, including half of those achieving only stable disease. Five patients had progressive disease, with four of these having extra-abdominal tumours and CTNNB1 mutations. Nine out of 37 female patients had ovarian dysfunction and eight patients discontinued treatment due to toxicity.
Digklia thinks that data reported so far are still insufficient to establish first-line nirogacestat for all patients with desmoid tumours. Due to the lack of comparative studies, it is still not possible to propose a definitive sequence of existing available therapies. In addition, data are pending on the optimal treatment duration. However, she notes that the preponderance of extra-abdominal tumours among patients progressing in the DeNi study is particularly instructive to help guide treatment decisions. “The highest risk of mortality is observed in patients with abdominal desmoid tumours who may benefit most from treatment with the oral gamma secretase inhibitor, especially those with bulky disease. Also, because the impact of nirogacestat on ovarian function remains a challenge, physicians may prefer to choose an alternative approach for women of child-bearing age that does not affect reproductive potential,” she says.
Digklia commends the authors for their dedicated effort in conducting the DeFi trial, which has played a key role in bringing this active agent to approval. Regarding the future of desmoid tumour management, she concludes: “We have existing active agents, including sorafenib, with established efficacy, and new chemotherapy strategies are also showing benefits, such as the administration of three cycles of nab-paclitaxel, which has good efficacy and provides effective pain relief for most patients, and which is tolerable over a short course (Nat Commun. 2022;13:6278). An exciting new approach being investigated is the combination of nirogacestat plus cryoablation (NCT05949099), which has the potential to make a lasting difference to patients.”
Programme details
Kasper B, et al. Long-term nirogacestat treatment in adult patients with desmoid tumors: Updated safety and efficacy from the phase III DeFi trial. ESMO Sarcoma and Rare Cancers Congress 2025, Abstract 67MO
Mini Oral Session 1, 20.03.2025, h. 10:30 – 12:00, Hall A
Kubicek P, et al. Deni study: Real world data on the use of nirogacestat (Ni) in patients (Pts) with desmoid tumors in the French Sarcoma Group. ESMO Sarcoma and Rare Cancers Congress 2025, Abstract 83P
Poster Display Session, 21.03.2025, h. 17:50 – 18:45, Villa Ciani