A study reports a disease-free survival benefit with six years of maintenance therapy with imatinib compared to the 3-year standard of care
As presented at today’s ESMO Virtual Plenary, a first analysis from the IMADGIST trial supports a 6-year duration of adjuvant therapy with the tyrosine kinase inhibitor (TKI) imatinib for patients with localised, completely resected gastrointestinal stromal tumour (GIST) with KIT expression and a high risk of tumour recurrence.
IMADGIST is a multicenter open-label, randomised, phase III study evaluating the maintenance of imatinib for 3 more years (6-year arm) at the last dose taken prior to randomisation (either 300 or 400 mg/d), compared to interruption of imatinib treatment (3-year arm) from the day of randomisation in 136 patients with a risk of tumour recurrence ≥35% according to National Comprehensive Cancer Network Task Force on GIST (NCCN) risk classification.
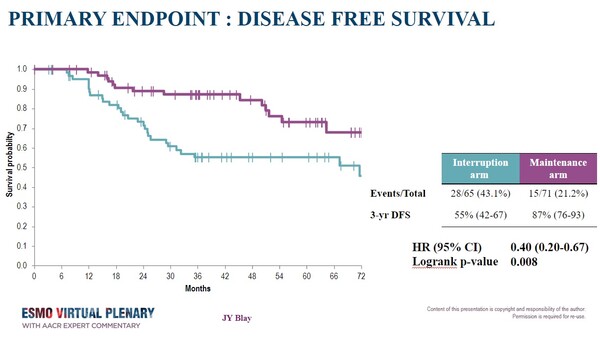
Earlier this year, first data from the study were presented at the ESMO Sarcoma and Rare Cancers Congress 2024, reporting that a prolonged use of adjuvant imatinib significantly improved 3-year disease-free survival (DFS) compared to the standard therapy (87% vs 55%, respectively; HR, 0.40; 95% CI: 0.20-0.67; P= 0.008).
Additional data, although immature, show a median progression-free survival (PFS) of 36 months after imatinib reintroduction in the 3-year arm, and no significant differences in other secondary endpoints including time to imatinib resistance and overall survival. “These results need to be further confirmed by mature overall survival data at a long-term follow-up”, says Prof. Hans Gelderblom, Leiden University Medical Center, The Netherlands commenting on the analysis presented.
For patients with high-risk, completely resected GIST, the ESMO-EURACAN-GENTURIS guidelines recommend that imatinib is given for 3 years as adjuvant therapy (Ann Oncol. 2022;33:20–33), based on results from the SSGXVIII/AIO trial demonstrating a survival benefit of imatinib treatment maintenance compared to a one-year therapy (J Clin Oncol. 2016;34:244–250). According to Gelderblom, the choice to pursue a prolonged regimen depends on the risk-benefit profile of the adjuvant therapy.
“In the first analysis of IMADGIST, main imatinib-related toxicities such as muscle cramps, diarrhoea, abdominal pain, asthenia, myalgia, and periorbital edema, considerably increased in the 6-year arm, suggesting that the DFS benefit of the prolonged adjuvant therapy is not without impact to patients,” he concludes.