Nirogacestat improves progression-free survival in a randomised trial performed in desmoid tumours
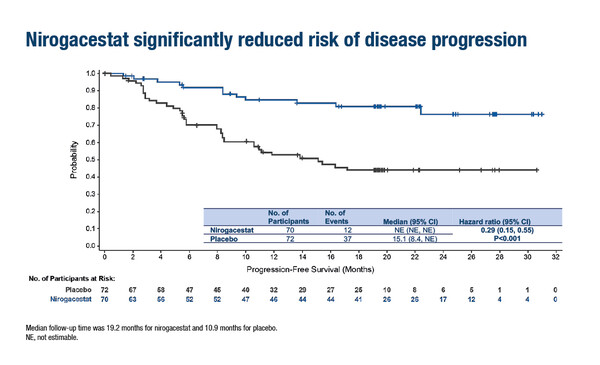
According to results from the phase III, global, double-blind DeFi trial presented at ESMO Congress 2022, the oral gamma secretase inhibitor (GSI) nirogacestat led to a significant 71% reduction in the risk of progression compared with placebo (hazard ratio for progression-free survival [PFS] 0.29; 95% confidence interval 0.15–0.55; p<0.001) in 142 adults with progressing desmoid tumours (DTs) (LBA2). In this 1:1 randomised trial, progressing DT was defined according to RECIST v1.1 and patients were stratified by tumour target location (intra-/extra-abdominal). The primary endpoint of PFS was assessed per blinded independent central review. Treatment with nirogacestat also significantly increased the objective response rate (41% versus 8%; p<0.001) and reduced the median time to response (5.6 months versus 11.1 months).
“These results are clinically meaningful and consistent with the biological mechanism of nirogacestat,” says Dr Alessandro Gronchi from Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy. “The benefit of nirogacestat shown in the DeFi trial opens the way for using this agent in DTs when an active treatment is needed. Over the past few years, the initial management of DT has shifted from surgery to active surveillance, given the spontaneous growth arrest and regression seen in up to 20% of patients (Clin Cancer Res. 2022:Feb). When growth arrest and regression do not occur spontaneously or there is a clinical need to induce them promptly, nirogacestat is an attractive option given its high activity and safety profile.”
In the context of the current medical treatment armamentarium, Gronchi continues, “The first published phase III study, which was of similar design to the DeFi trial, demonstrated that the kinase inhibitor sorafenib significantly prolonged PFS over placebo in patients with progressive, symptomatic or recurrent DT, with a hazard ratio for progression or death of 0.13 (N Engl J Med. 2018;379:2417–2428). However, although used in North America, sorafenib is not approved in Europe so when targeted therapy is needed, the TKI pazopanib is used, based on results from a phase II non-comparative trial of pazopanib or vinblastine plus methotrexate (Lancet Oncol. 2019;20:1263–1271). The other medical option widely used in the disease is the combination of low-dose methotrexate and vinca alkaloids. This treatment is known to be active, but its effect is slower and the acute side-effects may be more pronounced. In addition, it has never been studied formally. Conventional anthracycline-based chemotherapy is also active, but it comes at a significantly higher cost of side-effects (Eur J Cancer. 2020;127:96–107). Hence, nirogacestat may become the first-line treatment in the disease.”
DTs are a rare disease with an unpredictable disease course characterised by periods of spontaneous growth arrest followed by regression. Prospective cohort studies have shown that during active surveillance, approximately 30% of DTs will initially regress (Ann Surg. 2022:Feb) and another 20% of patients will spontaneously regress after initial progression (Clin Cancer Res. 2022:Feb). Gronchi says, “The design of the trial to include patients only with documented progression before study entry is important because, in order to understand the efficacy of the agent, you need to be sure the tumour is in the progressive phase, otherwise it becomes more complicated to distinguish whether tumour growth arrest/regression is due to the direct effect of the treatment or the natural course of the disease.”
Regarding patient-reported outcomes, nirogacestat was associated with early and sustained statistically and clinically significant improvements in all pre-specified measures – including physical/role functioning and health-related quality of life (QoL) – using DT-specific and general QoL tools. Toxicity of nirogacestat was manageable: the most frequent adverse events were diarrhoea (84%), nausea (54%) and fatigue (51%), and 95% of events with nirogacestat were grade 1–2. Ovarian dysfunction resolved in 20 (74%) of 27 women in the nirogacestat arm who developed it. Gronchi comments, “DTs rarely impact survival or life expectancy, unless located at a critical site, but they can impact quality of life. The effectiveness of nirogacestat in the DeFi trial is impressive, not only in terms of efficacy but also in terms of safety, tolerability and QoL. The only concern with GSIs may relate to fertility issues, and we need more data to confirm to what extent such effects are reversible.”
Looking to the future, Gronchi concludes, “The most important next step in the use of these agents in DTs is to understand optimal treatment duration and when to stop treatment. In the phase III trials, patients received treatment until progression but in a real-life setting, it is not feasible for patients to continue to receive treatment indefinitely at the risk of side-effects, given the lack of metastatic potential of DTs and the favourable course observed once regression has been achieved.”
Abstract presented:
Kasper B, et al. DeFi: A phase 3, randomized controlled trial of nirogacestat versus placebo for progressing desmoid tumors (DT). ESMO Congress 2022, LBA2
Presidential Symposium I, 10.09.2022, h. 16:30 – 18:00, Paris Auditorium