Detecting ctDNA and a simple four-component scoring system showed some predictive value, but require prospective validation
Arising from the adrenal gland, phaeochromocytomas and paragangliomas (PPGLs) and adrenocortical carcinoma (ACC) are extremely rare – incidences are 2–8 and ~0.5–2 per million per year, respectively – and they are characterised by heterogeneity in outcomes (Ann Oncol. 2020;31:1476–1490). Presentations at the ESMO Sarcoma and Rare Cancers Congress 2025 (Lugano, 20–22 March) highlight two novel and promising approaches that were able to stratify patients into distinct categories related to survival rates and associated with treatment responses.
“Patients with metastatic PPGL (mPPGL) and ACC can have varying disease courses, but only limited information is available on prognostic factors due to difficulties in gathering adequate patient numbers to obtain generalisable data,” comments Prof. Alfredo Berruti from the University of Brescia, Italy.
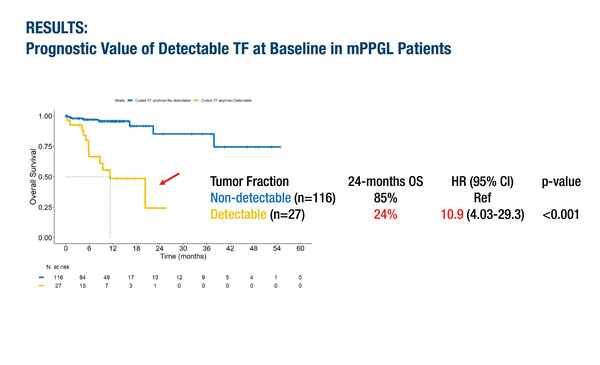
In the first presentation, plasma circulating tumour DNA (ctDNA) tumour fraction (TF) was demonstrated to be a clinically relevant biomarker for prognostication in mPPGL (Abstract 37O). When plasma samples were analysed from 143 patients included in the European Network for the Study of Adrenal Tumours (ENSAT) and American-Australian-Asian Adrenal Alliance (A5), ctDNA TF was detected in 19% of cases and of these, 91% had progressive disease and/or died during follow-up. In contrast, 71% of patients with undetectable ctDNA TF had stable disease. Detectable ctDNA TF was associated with a 24-month overall survival (OS) rate of 24% compared with 85% for non-detectable ctDNA TF (hazard ratio [HR] 10.9; 95% confidence interval [CI] 4.03–29.3; p<0.001). When the detectable ctDNA TF group was subdivided, HRs for OS were 5.38 (95% CI 1.51–19.2; p=0.009) with TF 2–10% and 27.3 (95% CI 8.43–88.7; p<0.001) with TF >10%. The researchers found that patients with detectable ctDNA TF had higher 12-month OS rates if treated with surgery and evidence-based targeted therapies compared with chemotherapy or watch-and-wait management (70% versus 20%; HR 8.35; 95% CI 1.75–39.9; p=0.008).
“This retrospective analysis provides the first evidence of the usefulness of ctDNA to predict prognosis and guide treatment in mPPGL,” notes Berruti, who explains: “There has been a real unmet need for markers that help to determine an individual patient’s mPPGL phenotype, which can range from indolent disease treated with active surveillance or local therapies, to intermediate disease managed with radionuclides or antiangiogenic therapies, to very aggressive disease treated with chemotherapy.” He highlights that elevated levels of plasma methoxytyramine, a circulating marker of dopaminergic differentiation, have previously been linked to metastatic likelihood in PPGL (Eur J Cancer. 2012;48:1739–1749) and suggests that evaluating methoxytyramine levels and ctDNA may reveal possible interactions. “In any case, serum methoxytyramine and ctDNA provide distinct and complementary information, and are two valuable prognostic parameters that deserve to be tested together prospectively,” he says.
A second study, also from ENSAT, developed a new scoring system to stratify patients with ACC by outcomes and response to specific systemic therapy regimens (Abstract 38O). In a multivariable analysis of 418 patients, tumour burden, cortisol levels, Eastern Cooperative Oncology Group performance status (ECOG PS) and neutrophil-to-lymphocyte ratio (NLR) were found to be significantly associated with survival. The researchers proposed the ‘BUCEN’ scoring system: tumour BUrden (score of 0, 1 or 2 based on tumour size, local recurrence and metastases), Cortisol excess (score of 1 if present), ECOG PS (score of 0, 1 or 2 for ECOG PS 0, 1 or 2–3, respectively) and NLR (score of 1 if ≥5). In patients who received mitotane monotherapy, a BUCEN score of 3 or more was associated with significantly shorter OS (HR 3.05; 95% CI 2.06–4.51; p<0.01) and shorter time-to-progression (HR 2.68; 95% CI 1.81–3.98; p<0.01). Similar results were observed in patients who received etoposide plus cisplatin with or without doxorubicin and with or without mitotane, or second-line options (gemcitabine plus capecitabine with or without mitotane, or temozolomide plus mitotane).
“Metastatic ACC is often very aggressive and current treatments have limited efficacy, but there is a small proportion of patients in whom treatments are effective or who have an indolent disease course. Early identification of these two categories is important for therapeutic planning and it seems the BUCEN score, by encompassing four easy-to-measure markers, is able to do this,” says Berruti. Mitotane levels within the target range of 14 mg/L or above have been shown to be a predictor of mitotane effectiveness (J Clin Endocrinol. 2011;96:1844–1851) and Berruti thinks it would be interesting to evaluate whether an interaction exists between BUCEN scores and mitotane serum levels.
He concludes, “It is only by collaborations like ENSAT and A5 that studies such as these can be conducted, but, in both cases, prospective validation is the next step to overcome the limitations of retrospective analyses and to help bring precision medicine to these extremely rare malignancies.”
Programme details
Toledo RD, et al. Plasma circulating tumor DNA (ctDNA) as a clinically relevant biomarker for disease monitoring and prognostication in metastatic pheochromocytoma and paraganglioma (mPPGL). ESMO Sarcoma and Rare Cancers Congress 2025, Abstract 37O
Proffered Paper Session, 21.03.2025, h. 10:30 – 12:00, Hall A
Mangone A, et al. Clinical predictors of treatment response in advanced adrenocortical carcinoma: A multicentre ENSAT study. ESMO Sarcoma and Rare Cancers Congress 2025, Abstract 38O
Proffered Paper Session, 21.03.2025, h. 10:30 – 12:00, Hall A