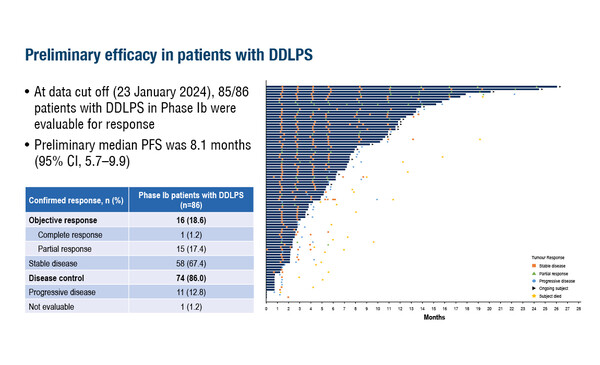
An early-phase trial shows the efficacy of brigimadlin in dedifferentiated and well-differentiated liposarcomas, but confirmation from phase III results is still awaited
According to results from a phase Ia/b trial presented at the ESMO Sarcoma and Rare Cancers Congress 2024 (Lugano, 14–16 March), the MDM2–p53 antagonist, brigimadlin, was associated with a preliminary median progression-free survival (PFS) of 8.1 months in 85 response-evaluable patients with MDM2-amplified advanced dedifferentiated liposarcoma (DDLPS) (Abstract 56O). An objective response rate (ORR) of 18.6% comprised 1 complete response and 15 partial responses (PRs), and the disease control rate (DCR) of 86% included an additional 58 patients with stable disease. Grade ≥3 treatment-related adverse events were reported in 43.0% of patients and were most commonly thrombocytopenia (23.3%) and neutropenia (20.9%). Six patients (7.0%) discontinued treatment due to adverse events.
In another presentation from a different cohort of patients in the same phase Ia/b trial, a preliminary median PFS of 25.1 months was observed in 31 response-evaluable patients with MDM2-amplified well-differentiated LPS (WDLPS) (Abstract 61MO). A DCR of 95% comprised 3 PRs and 16 patients with stable disease.
“Patients with DDLPS generally respond poorly to standard first-line doxorubicin treatment, with disease stabilisation being the most common positive outcome, so the fact that 16 patients achieved a response with brigimadlin is particularly encouraging,” comments Dr César Serrano from Vall d'Hebron Institute of Oncology (VHIO), Barcelona, Spain. “Also important,” he continues, “is that brigimadlin appears to be well-tolerated at this stage. Toxicity, mainly thrombocytopenia, has been a challenge in the development of MDM2 inhibitors, but in this study, discontinuations due to adverse events were low.”
“However,” cautions Serrano, “we have seen a number of MDM2 inhibitors with good early-phase data fail to reproduce efficacy in later-phase trials. Only last year, the phase III MANTRA trial reported that milademetan did not improve PFS over the chemotherapy agent trabectedin in DDLPS. Because of this, the sarcoma community is holding its breath waiting for the results of the phase III Brightline-1 trial (NCT05218499), comparing brigimadlin and doxorubicin first-line in DDLPS, which is due to report this year.” The ongoing single-arm phase III Brightline-4 trial may provide further insights into the use of brigimadlin in treatment-naïve or previously treated DDLPS (NCT06058793).
“The clinical significance of the efficacy results in WDLPS require more contextualisation,” suggests Serrano. “WDLPS is generally indolent and can be treated successfully with resection. However, WDLPS often coexists with DDLPS and it is the latter, aggressive component that drives poor prognosis. It is useful to know that brigimadlin treatment for DDLPS may also shrink the WDLPS component of the disease to facilitate surgery,” he concludes.
Abstracts discussed:
Schöffski P, et al. A phase Ia/Ib study of the MDM2–p53 antagonist brigimadlin (BI 907828): safety and efficacy in patients with dedifferentiated liposarcoma. ESMO Sarcoma and Rare Cancers Congress 2024, Abstract 56O
Proffered Paper Session 2, 14.03.2024, h. 16:15 – 17:45, Hall A
Reichardt P, et al. Phase Ia/Ib study of the MDM2–p53 antagonist brigimadlin (BI 907828) in advanced solid tumours: overall safety, and efficacy in patients (pts) with well-differentiated liposarcoma (WDLPS). ESMO Sarcoma and Rare Cancers Congress 2024, Abstract 61MO
Mini Oral Session, 15.03.2024, h. 13:00 – 14:00, Hall A