Impressive results from the Chinese LUMINET-1 phase III study confirm and extend previous findings in Western patients
As presented at the ESMO Asia Congress 2025 (Singapore, 5–7 December), the LUMINET-1 study reported highly positive findings, confirming that 177Lutetium (Lu)-DOTATATE has a place in the treatment not just of grade 2 or 3 gastroenteropancreatic neuroendocrine tumours (GEP-NETs) – as reported in the NETTER-2 combination trial in mainly Western patients (Lancet. 2024;403:2807–2817) – but also in lower grade GEP-NETs (LBA3).
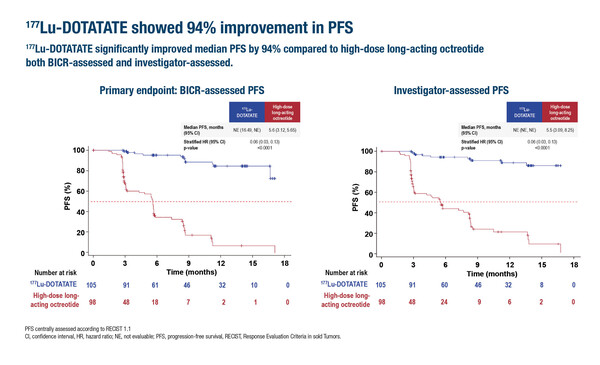
In the study, peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE led to a 94% improvement in the primary endpoint – progression-free survival (PFS) – compared with high-dose long-acting octreotide (hazard ratio [HR] 0.06; 95% confidence interval [CI] 0.03–0.13; p<0.001) among 203 treated patients with unresectable advanced grade 1 or 2 somatostatin receptor-positive GEP-NETs who had progressed on standard-dose octreotide.
“NETs used to be thought of as a rare disease in Asia, but today the incidence is rising and many new cases are GEP-NETs,” says Prof. Stephen Chan from the Chinese University of Hong Kong, Hong Kong SAR, China. “The trial demonstrates convincingly that 177Lu-DOTATATE is effective as monotherapy, the results being particularly impressive given the hazard ratio of 0.06.” Median PFS was only 5.6 months in the control arm at a 9-month median follow-up, but was not reached after a median of 10.6 months in the experimental arm.
The PFS benefit of 177Lu-DOTATATE was evident across pre-specified demographic and prognostic subgroups, including age, sex, primary tumour location and number of prior lines of therapy. 177Lu-DOTATATE also improved rates of objective response (40.0% versus 2.0%) and disease control (93.3% versus 62.2%). While overall survival data are immature, there was a trend towards a considerable reduction in the risk of death with 177Lu-DOTATATE (HR 0.17; 95% CI 0.04–0.79; p=0.0106).
Tolerability results did not reveal any unexpected findings. Grade ≥3 treatment-related adverse events (TRAEs) were reported in 39.0% of patients receiving 177Lu-DOTATATE and 4.1% receiving octreotide. The most common TRAEs with PRRT were haematological events, including reductions in the counts of white blood cells (53.3%), lymphocytes (44.8%) and platelets (43.8%).
Chan points out some of the challenges facing PRRT in the clinic, including the practicalities of implementing therapy, which requires close collaboration between oncologists and nuclear medicine specialists along with a high degree of expertise, and the need for more information on the absolute magnitude of survival benefit and long-term toxicity. Looking to future radionuclide therapies, he notes: “Investigation of alpha particle-emitting agents, such as 225Actinium (225Ac), which have the potential for greater tumour penetration than beta-emitting agents like 117Lu, is a very exciting direction for PRRT.” Preliminary completion of the phase III ACTION-1 study of 225Ac-DOTATATE in patients progressing on 177Lu-based PRRT is expected by the end of 2025 (NCT05477576). Targeted therapies, such as the multitargeted tyrosine kinase inhibitor zanzalintinib, which is under phase III investigation in the STELLAR-311 trial (NCT06943755), may be another possible option for patients whose tumours progress on previous treatment.
Programme details:
Chen J, et al. 177Lu-DOTATATE versus high-dose long-acting octreotide for somatostatin receptor-positive advanced GEP-NETs: A multicenter, randomised, open-label, positive-controlled phase III study. ESMO Asia Congress 2025 - LBA3