Positive findings are presented for advanced Merkel-cell carcinoma plus phaeochromocytoma and paragangliomas, while negative findings are equally important to guide future research
“Innovating to improve the treatment of rare cancers is extremely difficult due to challenges including gaining funding, recruiting sufficient patients, disease heterogeneity and overcoming regulatory barriers,” explains Dr Armelle Dufresne from the Centre Léon Bérard, Lyon, France, welcoming the various studies presented at the ESMO Congress 2025 (Berlin, 17–21 October) in the field of rare malignancies.
Encouraging findings come from immunotherapy, a therapeutic strategy that has recently produced positive outcomes in some rare tumours. Previously, PD-1 blockade has shown efficacy in patients with advanced Merkel-cell carcinoma (MCC) (N Engl J Med. 2016;374:2542–2552). A Late-Breaking presentation in Berlin described a trend towards positive results with pembrolizumab in the multicentre US EA6174 STAMP trial, the first randomised phase III report of adjuvant immunotherapy in MCC (LBA56, key results in the box below). A co-primary endpoint was relapse-free survival (RFS) and across 293 patients with resected MCC, 2-year RFS rates were 73% with pembrolizumab versus 66% with observation (hazard ratio [HR] for RFS 0.80; 95% confidence interval [CI] 0.53–1.22). “The number of patients recruited in the study is impressive and demonstrates that it is possible to conduct a randomised phase III trial in a rare cancer, even in the adjuvant setting,” notes Dufresne. Mature data on OS – the other co-primary endpoint – are not yet available and, if positive, this study may have a practice-changing impact.
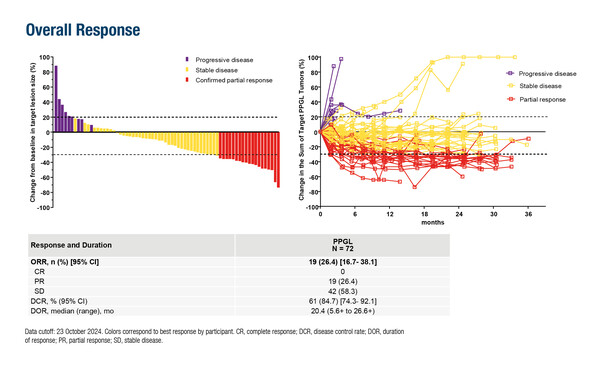
Impressive response and disease control rates were also demonstrated in the non-randomised phase II LITESPARK-015 trial of the hypoxia-inducible factor (HIF)-2α inhibitor, belzutifan, in patients with locally advanced or metastatic phaeochromocytoma and paragangliomas (PPGL) (Abstract 1705O). Among 72 patients with a median follow-up of 30.2 months, an objective response rate of 26.4% was reported with a disease control rate of 84.7%. Tumour efficacy findings were further supported by sustained reductions in total daily dose of antihypertensive medication and by stable or improved EORTC QLQ-C30 general health status/quality of life and physical functioning scores.
However, as Dufresne highlights, these results may not change practice due to the nature of the study design. “Regulators are often reluctant to consider results such as these from phase II and non-randomised trials, and this is a common issue in rare cancers,” she explains. Currently, no standard of care (SOC) treatment is available for the patients with PPGL, “so, when an innovative treatment emerges, it is important that patients get access as soon as possible. It is also heartening to see pharmaceutical funding for the trial, which is something to be encouraged to progress research in the field.” A shift from the traditional organ-based classification of cancers to a tumour-agnostic approach (Ann Oncol. 2024;35:936–953) and innovative trial design (e.g. basket trials) are emerging as opportunities that may deliver considerable benefits.
What to do next in the face of modest benefits in a phase II trial is another common issue associated with drug development for rare cancers. “Patient numbers are usually too low to support continuing development in a subgroup of the initial population. And while larger benefits may be seen if the agent is combined with another agent, there may be a reluctance to perform a randomised trial without a stronger signal for efficacy,” says Dufresne. An example of this is represented by not-so-encouraging results from the single-arm phase II ALPACA trial, which investigated avelumab in 23 evaluable patients with locally advanced or metastatic penile squamous cell carcinomas previously treated with, or unfit for, platinum-based chemotherapy (LBA37, key results in the box below). Best response was a partial response (PR) in 4 patients (17%) and stable disease in 1 patient (4%), yielding a clinical benefit rate of only 21%.
The challenge of interpreting subgroup data is reflected in an exploratory analysis of patients with chromophobe renal cell carcinoma (RCC) from the phase II SUNNIFORECAST trial, which compared ipilimumab plus nivolumab with SOC in 309 patients with non-clear cell RCC (Abstract 2595MO, key results in the box below). Numerical, but not significant, improvements in the primary endpoint of OS were observed in 59 patients with chromophobe RCC treated with ipilimumab plus nivolumab versus SOC (median [m]OS: 40.2 months versus 36 months; HR 0.77; 95% CI 0.37–1.63; p=0.502). Patients were not stratified by PD-L1 status but those with a combined positive score (CPS) ≥1 (n=19) appeared to have longer mOS with ipilimumab plus nivolumab than SOC (45.3 months versus 10.6 months; p=0.051), while patients with CPS <1 (n=36) showed a tendency towards longer mPFS with SOC (5.1 months versus 8.6 months; p=0.410). “Like more common tumours, there is heterogeneity of response in certain rare cancers, but interpreting subgroup analyses to support biomarker testing is difficult with such small patient numbers. Nevertheless, these data do appear to show a potential role for PD-L1 testing and subsequent benefit with immune checkpoint inhibition for patients with chromophobe RCC and CPS ≥1,” Dufresne observes.
In a field where research progresses at a slower pace than in other areas of oncology, negative findings may provide important learnings about what can be achieved and where further efforts are needed. This is the case with a randomised Chinese trial comparing intensity-modulated proton therapy (IMPT) and intensity-modulated carbon ion therapy (IMCT) in 52 patients with skull base chordoma or chondrosarcoma treated with curative intent (Abstract 656O, key results in the box below). For the primary endpoint of local progression-free survival (LPFS), 5-year rates were 75.3% with IMPT and 75.7% with IMCT (p=0.46), with no significant difference between tolerability profiles. “Local control of chordoma or chondrosarcoma at this specific skull location is important due to the heavy burden of symptoms. One might have thought that IMPT would be more focused with a higher dose delivered to the tumour but this did not appear to significantly affect LPFS,” says Dufresne.
She concludes: “Presenting and publishing data on rare cancers is challenging, particularly with negative findings, but it is essential, so that the incredible effort needed to run these trials is not unnecessarily duplicated. ESMO’s peer-reviewed journal ESMO Rare Cancers and ESMO Sarcoma and Rare Cancers Congress provide platforms for researchers to share their findings – positive and negative – to ensure they reach the wider scientific community.”
At a glance:
Mehnert JM, et al. ECOG-ACRIN EA6174: STAMP: Surgically treated adjuvant Merkel cell carcinoma with pembrolizumab, a phase III trial. ESMO Congress 2025 - LBA56
- Pembrolizumab vs observation (n=293):
- 2-y RFS: 73% vs 66% (HR 0.80; 90% CI 0.53–1.22; log-rank p=0.105)
- DMFS 40% vs 60%; HR 0.58; 95% CI 0.35–0.94; p=0.032
- Grade ≥3 TRAEs: 31% vs 4%; 1 grade 5 pneumonitis in pembrolizumab arm
Jimenez C, et al. LITESPARK-015: Belzutifan in advanced pheochromocytoma and paraganglioma. ESMO Congress 2025 - Abstract 1705O
- Patients enrolled and received belzutifan:
- DCR: 84.7%
- mPFS: 22.3 mo
- mOS: NR
- Grade ≥3 TRAEs: 46% (anaemia, 22%; hypoxia, 10%)
Sridhar SS, et al. A phase II study of avelumab in locally advanced or metastatic penile cancer patients unfit for platinum-based chemotherapy or progressed on or after platinum-based chemotherapy (ALPACA). ESMO Congress 2025 - LBA37
- Patients evaluable for efficacy (n=23):
- CBR > 24 wk: 21%
- mPFS: 1.7 mo
- mOS: 3.9 mo
- Grade ≥3 TRAEs: infusion reaction, fatigue, anaemia and diarrhoea (4% each)
Ahrens M, et al. Exploratory analysis of chromophobe renal cell carcinoma in the SUNNIFORECAST trial comparing ipilimumab plus nivolumab vs standard of care as first line treatment. ESMO Congress 2025 - Abstract 2595MO
- Ipilimumab plus nivolumab vs SOC (n=59)
- mOS: 40.2 mo vs 36.0 mo; p=0.502
- mOS in 19 pts with CPS ≥1: 45.3 mo vs 10.6 mo; HR 0.27; p=0.051
- mPFS in 36 pts with CPS <1: 5.1 mo vs 8.6 mo; HR 1.41; p=0.410
Qingting H, et al. Proton versus carbon ion radiotherapy in skull base chordoma and chondrosarcoma: Initial clinical outcomes from a phase II randomized trial. ESMO Congress 2025 - Abstract 656O
- IMPT vs IMCT (n=52)
- 5-y LPFS: 75.3% vs 75.7%; p=0.46
- Grade 1–2 acute mucositis: 15.4% vs 19.2%
- Grade 1–2 acute xerostomia: 15.4% vs 3.8%
- Grade 1–2 acute alopecia: 3.8% vs 11.5%