Rivoceranib after prior therapy and peri-operative pembrolizumab demonstrates promising efficacy but it is not superior to the current standard therapy
“It is encouraging to see clinical trials in this rare tumour type, which is often neglected in oncology research. However, the results discussed at the ESMO Congress 2025 (Berlin, 17–21 October) must be taken with caution,” says Prof. Nicolas Girard from the Institut Curie, Paris, and the Paris Saclay University, France, commenting on two phase II trials in metastatic and locally-advanced thymic epithelial tumours at a Proffered Paper session on less common thoracic malignancies, including thymic cancer.
Both the selective VEGFR2 inhibitor, rivoceranib, as second- or later-line therapy (LBA105, key results in the box below) and peri-operative pembrolizumab in addition to standard therapy (Abstract 2967O, key results in the box below), in fact, showed acceptable efficacy, which was not superior to that reported for the current standard of care. Metastatic thymic epithelial tumours, comprising thymomas and thymic carcinomas, are aggressive, rare malignancies with limited treatment options beyond platinum-based chemotherapy (Mediastinum. 2024;8:33). Agents with anti-angiogenic properties, lenvatinib and sunitinib, are currently recommended by ESMO Clinical Practice Guidelines (Ann Oncol. 2015(Suppl 5):v40–v55) and are considered standard of care for recurrent/metastatic disease.
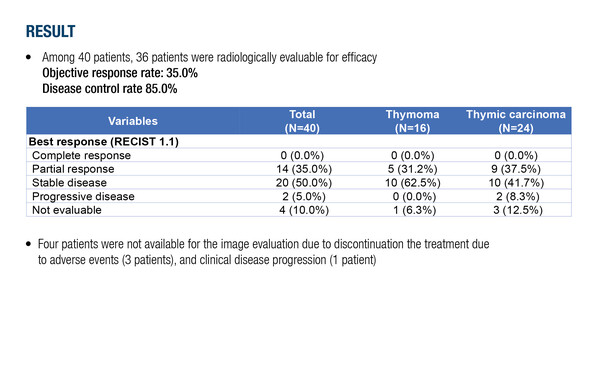
In a multicentre, prospective, single-arm phase II study, rivoceranib was associated with an objective response rate (ORR) of 35.0% in 40 previously treated patients with metastatic thymic epithelial tumours. “The response rate was relatively high in this advanced setting, similar to that previously demonstrated with lenvatinib in a phase II study of advanced/metastatic thymic carcinomas (ORR of 38%) (Lancet Oncol. 2020;21:843–850),” says Girard.
He notes that the rivoceranib study included patients with thymic carcinoma or thymoma, while the lenvatinib study was conducted in patients with thymic carcinoma only. “It is difficult to see how this treatment can be differentiated from lenvatinib. Rivoceranib seems to be effective in thymomas where historically only sunitinib has shown activity. It would be useful to see additional data with longer follow-up, including overall survival and efficacy by histology,” Girard adds. Median follow-up was 3.7 months, compared with 15.5 months in the lenvatinib study. Progression-free survival was not reached (versus ~9 months with lenvatinib) and median duration of response was 1.7 months. Safety was as expected for an anti-angiogenic agent, being hypertension the most commonly reported adverse event (47.5%).
In locally advanced thymic tumours, the goal is to achieve complete resection, and pre-operative chemotherapy is commonly used to facilitate surgery when there is no high likelihood of R0 with surgical resection. “This is very different to lung cancer, for example, where we give peri-operative chemotherapy to treat micro-metastases and reduce the risk of recurrence or prolong survival. With thymic tumours, prognosis is not driven by response to chemotherapy but by achieving an R0 resection,” explains Girard.
In a second phase II study presented at the ESMO Congress, the addition of pembrolizumab to peri-operative chemotherapy in 40 patients with locally advanced thymic epithelial tumours was associated with an ORR of 57.5% at a median follow-up of 27.5 months. Of 28 patients who received surgery, R0 resection was achieved in 71.4%. Major pathologic response and pathologic complete response (pCR) were observed in 46.4% and 17.9% of patients, respectively. “The resection rate with pembrolizumab is typical of that achieved with chemotherapy alone in clinical practice, so is in line with what we might expect, but not better,” Girard remarks.
Immunotherapy is associated with a high risk of toxicity in patients with thymic tumours, especially in those with thymoma. Because the thymus is involved in the regulation of autoimmunity, adverse events occur at a much higher rate than in other cancers (Cancers (Basel). 2022;15:289). “Patients can be at risk of severe toxicities such as myocarditis, so we are very cautious about the use of immunotherapy in these patients, especially in the curative setting,” says Girard. During the study, 5 patients with thymoma experienced grade 4 adverse events (myocarditis; neutropenia; and aspartate transaminase/alanine transaminase elevation), and an additional 2 patients with thymoma died from myocarditis. He adds, “The fact that 5% of patients treated with curative intent died seems risky and suggests that this treatment strategy should not be applied to patients with thymoma.”
While the data appear promising, it is unclear whether the efficacy of pre-operative chemotherapy is improved with the addition of immunotherapy. “The pCR rate seems relatively high but given the importance of R0 resection to patient outcome and the toxicity observed, the value of adding immunotherapy in this setting is not immediately apparent,” Girard concludes.
While research to improve patient outcomes continues in the field, an update to the ESMO Clinical Practice Guidelines in thymic epithelial tumours is in preparation.
At a glance:
Ahn M-J, et al. A phase II, open-label, single-arm, multi-center study of rivoceranib in patients with metastatic thymic epithelial tumor, KCSG LU23-09 (THRIVE). ESMO Congress 2025 - LBA105
- N=40; n=36 evaluable for radiological efficacy
- Median follow-up: 3.7 mo
- ORR: 35.0% (14 PR); DCR: 85.0%
- Median change in tumour volume: -19.8% (range -69.7 to +50.7)
- mPFS: NR; mOS: NR
- Discontinuation due to TRAEs: n=3
Park S, et al. Perioperative pembrolizumab for locally advanced thymic epithelial tumors. ESMO Congress 2025 - Abstract 2967O
- N=40
- Median follow-up: 27.5 mo
- ORR: 57.5%
- Surgery: n=28
- R0 resection rate: 71.4%
- MPR: 46.4%; pCR: 17.9%
- mDFS: 49.3 mo
- Median change in tumour volume: -35.6% (range -1.2 to -79.5)
- Discontinuation due to TRAEs: n=12 (n=8 pre-surgery)