Disappointing results were reported from the phase III MANTRA trial, but biomarker-based approaches still offer hope for MDM2 inhibition in solid tumours
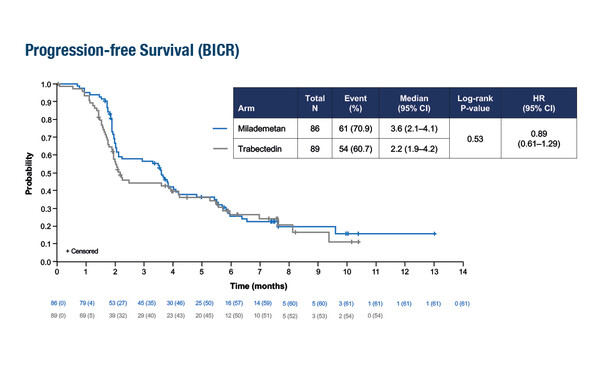
Results presented at the ESMO Congress 2023 (Madrid, 20–24 October) revealed no significant difference in median progression-free survival (PFS) between the MDM2 inhibitor milademetan and the chemotherapy agent trabectedin (3.6 months versus 2.2 months; hazard ratio [HR] 0.89; 95% confidence interval [CI] 0.61–1.29) in unresectable/metastatic dedifferentiated liposarcomas (DDLPS) (LBA89). The trial involved 175 patients who had progressed on at least one anthracycline regimen. Median overall survival was 9.5 months with milademetan and 10.2 months with trabectedin (HR 1.27; 95% CI 0.80–2.04). Overall confirmed response rates were 4.7% for milademetan and 3.4% for trabectedin (p=0.667) when assessed by blinded independent central review and 10.5% and 2.2%, respectively, when assessed locally by investigators (p=0.026).
The most common treatment-related adverse events (TRAEs) with milademetan were nausea (62.8% versus 58.2% with trabectedin), thrombocytopenia (60.5% versus 24.1%) and neutropenia (41.9% versus 35.4%). The most common grade 3–4 milademetan TRAEs were thrombocytopenia (39.5% versus 13.9%), neutropenia (25.6% versus 25.3%) and anaemia (11.6% versus 17.7%). There were no grade 5 TRAEs with milademetan but two with trabectedin. Treatment-emergent AEs leading to dose reduction were more common with milademetan than trabectedin (44.2% versus 29.1%, respectively), but those leading to discontinuation were less frequent (11.6% versus 19.0%, respectively). The authors concluded that further work is required to identify patients who are most likely to benefit from milademetan.
Although milademetan monotherapy did not provide clinical benefit in this population of patients with DDLPS, the strategy of restoring or stabilising p53 apoptotic function via MDM2 inhibition could prove useful in some solid tumour settings. Biomarker-based targeting of treatment is being investigated in the ongoing phase II, tumour agnostic, MANTRA-2 basket trial of milademetan in solid tumours with MDM2 amplification and wild-type TP53 (NCT05012397), with promising interim results and final results expected in 2024. Combination therapy is another avenue for exploration.
Abstract discussed:
Jones RL, et al. Efficacy and safety findings from MANTRA – a global, randomized, multicenter, phase 3 study of the MDM2 inhibitor milademetan vs trabectedin in patients with dedifferentiated liposarcomas. ESMO Congress 2023, LBA89
Proffered Paper Session – Sarcoma, 22.10.2023, h. 08:30 – 10:00, Valencia Auditorium – Hall 10