The PODIUM-303 study shows practice-changing results for patients with unresectable locally recurrent or metastatic squamous cell carcinoma of the anal canal
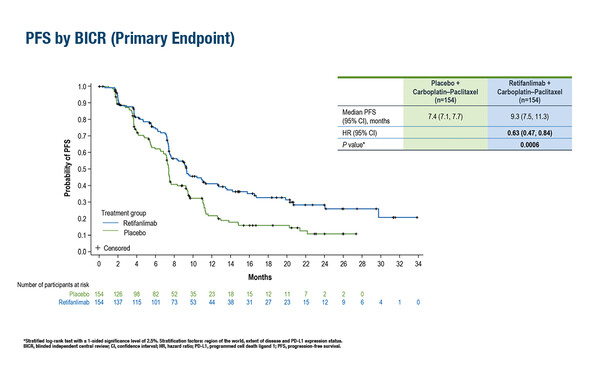
The phase III POD1UM-303 (InterAACT 2) study met its primary endpoint, with the anti-PD-1 immunotherapy retifanlimab plus standard of care (SoC) chemotherapy demonstrating significantly longer median progression-free survival (PFS) compared with chemotherapy alone in patients with previously untreated, locally recurrent or metastatic squamous cell carcinoma of the anal canal (SCAC; 9.3 months versus 7.4 months; hazard ratio [HR] 0.63; 95% confidence interval [CI] 0.47–0.84; p=0.0006). Data from this practice-changing study were presented in a Presidential Symposium at the ESMO Congress 2024 (Barcelona, 13–17 September; LBA2).
“This is the largest randomised trial in metastatic unresectable anal cancer, so this is something new and significant. Moreover, it has shown benefit over the current SoC, which suggests that retifanlimab plus carboplatin and paclitaxel may become a new SoC in SCAC,” comments Prof. Dominik Modest from the Charité-Universitätsmedizin Berlin, Germany.
Retifanlimab monotherapy was previously associated with promising signs of efficacy in a phase II study of patients with platinum-refractory SCAC (ESMO Open. 2022;7:100529). In the current phase III trial, 308 patients were randomised 1:1 to 6 cycles of standard-dose carboplatin and paclitaxel plus either placebo or retifanlimab (500 mg every 4 weeks) for up to 1 year, with crossover permitted for those patients in the initial chemotherapy alone treatment arm.
Overall survival (OS) data were immature at data cut-off, but with a median OS follow-up of almost 15 months for retifanlimab and 13 months for chemotherapy, a strong trend of improved OS was observed in favour of the retifanlimab arm as compared with the chemotherapy alone arm (median OS: 29.2 months versus 23.0 months; HR 0.70; 95% CI 0.49–1.01; p=0.0273).
The treatment is reported to be ‘generally well tolerated, with safety being consistent with other chemotherapy plus checkpoint inhibitor regimens and the delivery of chemotherapy was not compromised by retifanlimab administration’. “While it will be interesting to see more detailed data on toxicity and also adherence, currently it seems there are no apparent drawbacks to this combination,” adds Modest.
Additional randomised controlled data are needed to demonstrate whether retifanlimab may have a role in earlier stages of SCAC. “Other future research goals may be to explore the intensification of immunotherapy in anal carcinoma as immune checkpoint inhibition appears to be surprisingly active in this setting,” concludes Modest. “An alternative chemotherapy backbone including three substances as a combination partner for retifanlimab could also be considered (Lancet Oncol. 2024;25:518–528; Lancet Oncol. 2018;19:1094–1106).”
Programme details
Rao S, et al. POD1UM-303/InterAACT 2: Phase 3 study of retifanlimab with carboplatin-paclitaxel (C-P) in patients (Pts) with inoperable locally recurrent or metastatic squamous cell carcinoma of the anal canal (SCAC) not previously treated with systemic chemotherapy (Chemo). ESMO Congress 2024, LBA2
Presidential Symposium I – Practice-changing trials, 14.09.2024, h. 16:30 – 18:15, Barcelona Auditorium – Hall 2