Studies explore the prognostic and predictive values of circulating DNA tumour fraction and single-cell RNA sequencing of peripheral blood mononuclear cells
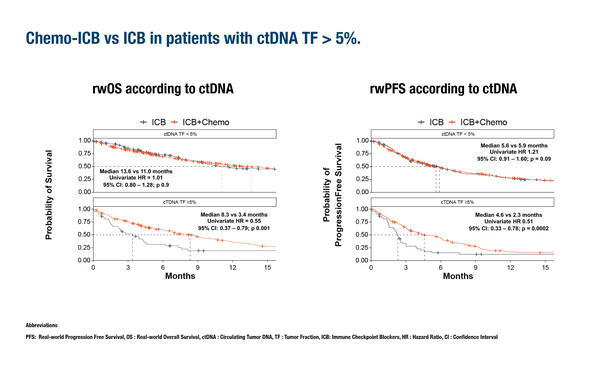
Circulating tumour DNA (ctDNA) tumour fraction (TF) – the fractional proportion of ctDNA relative to circulating cell-free DNA – is demonstrating increasing potential in cancer management (Ann Oncol. 2023;34:111–120). Now, results from a real-world analysis presented at the ESMO Immuno-Oncology Congress 2024 (Geneva, 11–13 December) provide evidence that ctDNA TF is a prognostic factor in patients receiving immune checkpoint inhibitors (ICIs) and could be useful in identifying which patients may benefit from intensification with chemotherapy (Abstract 4MO). Among 820 patients with advanced non-small cell lung cancer, a high ctDNA TF (≥10%) was associated with worse survival for patients receiving ICIs with and without chemotherapy. Notably, combination ICI and chemotherapy was associated with improved overall survival compared with ICI alone in patients with a ctDNA TF ≥5% (8.3 months versus 3.4 months; hazard ratio 0.55; 95% confidence interval 0.37–0.79; p=0.001) but not below this level. ctDNA TF showed a correlation with metabolic tumour volume. In addition, an association between TP53 and RB1 mutations and high ctDNA TF was observed after adjusting for metabolic tumour volume.
Prof. Christian Rolfo from the Ohio State University Comprehensive Cancer Center, Columbus, Ohio, USA, comments: “These findings add to the exciting array of new data on how ctDNA TF can improve on the value of ctDNA analysis and liquid biopsies. From initial prognostic investigations, we have now moved to the stage where ctDNA TF is being assessed for its ability to predict response.” He also cites work in which ctDNA TF was able to distinguish if a liquid biopsy was informative or not, which may be useful to guide whether prompt treatment initiation or follow-on tissue testing is appropriate (Clin Cancer Res. 2024;30:2452–2460).
Recent analysis of the phase III IMbassador250 trial in metastatic castration-resistant prostate cancer found that ctDNA TF was able to predict whether enzalutamide would provide survival benefit after abiraterone, complementing conventional prostate-specific antigen monitoring (Clin Cancer Res. 2024;30:4115–4122). “The possibility of using ctDNA TF to guide treatment selection is intriguing, but retrospective and real-world data now need to be backed up with prospective trials and standardisation of methodology,” notes Rolfo.
In another study, single-cell RNA sequencing of peripheral blood mononuclear cells was used to identify predictive markers for treatment benefit with TILT-123, a tumour necrosis factor-α- and IL-2-encoding oncolytic adenovirus (Abstract 6MO). When data were analysed from the phase I TUNIMO trial in patients with advanced solid tumours (Clin Cancer. 2024;30:3715–3725), those who benefited from TILT-123 had more defined cytotoxic immune cell expression profiles at baseline, including an increased presence of CD16+ monocytes and a reduced proportion of regulatory T-cells. Furthermore, the presence of cytotoxic NK cells and memory T-cells in peripheral blood positively predicted survival. Pre-existing B-cell immunity and specific T-cell receptor profiles at baseline were able to distinguish responders from non-responders.
Rolfo is encouraged by the use of innovative technologies to assess changes in immune cell signatures during treatment: “Using single-cell analyses to delineate mechanistic information and also to provide potential blood-based biomarkers for response at the phase I stage should help to support phase II development, which may be particularly valuable given the novel mode of action of this agent.”
He concludes: “The future of liquid biopsies in immuno-oncology is clearly bright. In addition to detecting pre-treatment mutations and genetic determinants of immunotherapy response, such as tumour mutation burden or microsatellite instability, two of the most interesting areas are the dynamic assessment of ctDNA over the course of treatment to improve risk stratification and the detection of minimal residual disease.”
Programme details
Dall'Olio FG, et al. Role of ctDNA tumor fraction to select immunotherapy based regimens in advanced non-small cell lung cancer. ESMO Immuno-Oncology Congress 2024, Abstract 4MO
Mini Oral Session 1, 12.12.2024, h. 08:30 – 10:00, Room C
Kudling T, et al. Single-cell analysis of peripheral blood mononuclear cells reveals therapy outcomes are associated with pre-existing immunity in patients treated with oncolytic adenovirus armed with TNFα and IL2. ESMO Immuno-Oncology Congress 2024, Abstract 6MO
Mini Oral Session 1, 12.12.2024, h. 08:30 – 10:00, Room C