Baseline and on-treatment changes in ctDNA may provide an early indication of response to experimental therapies
Two studies presented at the ESMO Targeted Anticancer Therapies Congress 2024 (Paris, 26–28 February) suggest that circulating tumour (ct)DNA could be a useful tumour-agnostic biomarker that may expedite the assessment of new therapeutics.
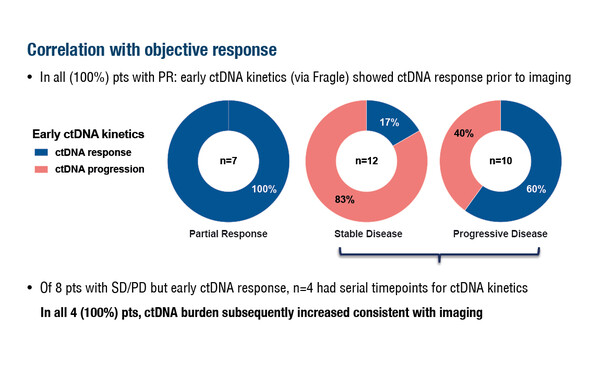
In the first study, among 34 patients with advanced cancer receiving treatment in phase I trials with targeted therapy (50%), immunotherapy (44%) or combination therapy (6%), all 7 achieving a partial response showed an early reduction in ctDNA burden (Abstract 85O). Eight patients with stable disease or progressive disease also showed an early ctDNA response. Further evaluation of serial timepoints in 4 of these patients showed that ctDNA burden subsequently increased and was consistent with imaging results. In this study, 102 samples (median 3 samples/patient) underwent low-pass whole-genome sequencing and ctDNA burden was quantified either using established methods (ichorCNA to measure DNA copy number) or novel techniques (Fragle to measure cell-free DNA fragmentation patterns). Fragle showed an enhanced limit of detection compared with ichorCNA, both at baseline (100% versus 53%) and on treatment (100% versus 41%).
In the second study, ctDNA mean variant allele fraction (mVAF) at baseline was associated with worse progression-free survival (PFS) (hazard ratio [HR] 1.06; 95% confidence interval [CI] 1.01–1.12; p=0.014) among 34 patients with advanced cancer taking part in 19 phase I trials (Abstract 86O). mVAF at baseline also improved the performance of a predictive model of progression based on age, sex, cancer type, sites of disease and prior lines of treatment (C-statistic improved from 0.57 to 0.65). Changes in mVAF showed significant increases in time points with radiological progression and decreases in those with response (2.40 versus −0.30; p=0.015). Patients with on-treatment early molecular reduction (baseline mVAF reduction >50% within 1.5 months) had improved PFS (HR 0.26; 95% CI 0.10–1.00; p=0.05). In this study, targeted next-generation sequencing was performed on 122 serial ctDNA samples (median 3 samples/patient) collected as part of the TARGET molecular profiling programme and response evaluation on radiological scans was conducted at each sample collection timepoint.
Prof. Christian Rolfo from The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA, comments: “These are preliminary data, but they provide a good indication that the dynamics of ctDNA may be useful as a surrogate for therapy outcomes. The demonstration that mVAF can be used to monitor changing ctDNA could help in predicting the risk of disease progression and in assessing early indications of response or resistance to treatment. In addition, ctDNA kinetics may act as a pharmacodynamic biomarker for therapeutic response, with significant implications for dose decision making in early phase trials.”
Rolfo concludes: “ctDNA analysis is non-invasive, rapid and relatively inexpensive, and results from studies like these further open up its potential to be more widely applied in early phase clinical trials.”
Abstracts discussed
Tan AC, et al. Low-pass whole genome sequencing (lpWGS) to quantify circulating tumor DNA (ctDNA) burden as a pharmacodynamic response biomarker in patients on early phase trials. ESMO Targeted Anticancer Therapies Congress 2024, Abstract 85O
Abstract Session 2, 27.02.2024, h. 15:45 – 17:15, Amphitheatre Bordeaux
Shohdy KS, et al. Serial monitoring of circulating tumor DNA (ctDNA) as a tumor-agnostic biomarker to inform clinical outcomes of patients receiving experimental cancer medicine. ESMO Targeted Anticancer Therapies Congress 2024, Abstract 86O
Abstract Session 2, 27.02.2024, h. 15:45 – 17:15, Amphitheatre Bordeaux