In the KEYNOTE-671, improvements in event-free survival and overall survival were observed after four years of follow up
Perioperative pembrolizumab plus neoadjuvant chemotherapy continued to provide improved event-free survival (EFS) and overall survival (OS) compared to neoadjuvant chemotherapy and surgery alone in early-stage non-small cell lung cancer (NSCLC), as a 4-year follow up of the KEYNOTE-671 study reported (LBA3).
The KEYNOTE-671 study is investigating the safety and efficacy of pembrolizumab in combination with platinum doublet neoadjuvant chemotherapy before surgery, followed by pembrolizumab alone after surgery in participants with resectable stage II, IIIA, and resectable IIIB (T3-4N2) NSCLC. Significant improvements in EFS (N Engl J Med. 2023 Aug 10;389(6):491-503) and OS (Lancet. 2024 Sep 28;404(10459):1240-1252) were previously reported leading to the approval of perioperative pembrolizumab in the Unites States, Europe, and other countries in this setting.
The long-term follow up results were presented at the ESMO Immuno-Oncology Congress held in Geneva in December 2024. A total of 797 patients were randomised to either receive pembrolizumab (n = 397) or a placebo (n = 400) plus chemotherapy for four cycles followed by surgery, and then adjuvant pembrolizumab or placebo for up to 13 cycles. Randomisation was stratified by disease stage (II vs III), PD-L1 tumour proportion score (<50% vs ≥50%), tumour histology (squamous vs nonsquamous), and geographic region (East Asia versus not East Asia).
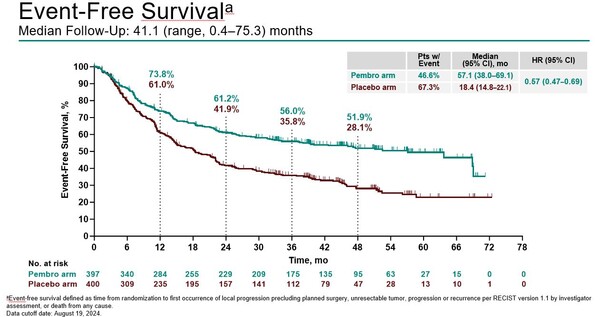
Median OS was not reached in either treatment arm (HR, 0.73; 95% CI, 0.58–0.92). After 4 years of follow up, median EFS was 57.1 months with pembrolizumab compared to 18.4 months with placebo (hazard ratio [HR] 0.57; 95% confidence interval [CI], 0.47–0.69) (Figure). The 48-month OS rate was 68.0% with pembrolizumab versus 56.7% with placebo, and the observed OS benefit was generally consistent across most patient subgroups.
“We have now six studies that have investigated the use of immunotherapy in the neoadjuvant or perioperative setting in NSCLC, and they are all positive”, said Prof. Marcello Tiseo, University of Parma, Parma, Italy, who was the invited discussant to comment on these findings in Geneva. He highlighted that the long-term follow up data from the KEYNOTE 617 were very similar to those reported from the CM 816 trial earlier in 2024 (J Clin Oncol 42, LBA8010-LBA8010(2024)), also adding that one of the most important question now is to clarify what is the best option for patients with resectable NSCLC, whether neoadjuvant or perioperative strategy. “Findings from the 4-year update of KEYNOTE-671 confirm the perioperative strategy as a standard of care for resectable NSCLC.”
Finally, safety was manageable across both arms. Grade ≥3 treatment-related adverse events (AEs) occurred in 45.2% patients in the pembrolizumab arm compared to 37.8% in the placebo arm; 4 (1.0%) and 3 (0.8%) treatment-related deaths occurred, respectively. Immune-mediated AEs and infusion reactions occurred in 26.0% in the pembrolizumab arm and 9.3% in the placebo arm, with 25 (6.3%) and 7 (1.8%), respectively, as grade ≥3.