Certain immunotherapy combinations that are effective in selected solid tumours may not be effective in lung cancer without targetable genetic alterations.
As presented at the ESMO Immuno-Oncology Congress 2023 (Geneva, 6–8 December), the combination of lenvatinib and pembrolizumab with/without chemotherapy did not prolong survival in previously untreated or treated patients with non-small-cell lung cancer (NSCLC) without targeted genetic alterations.
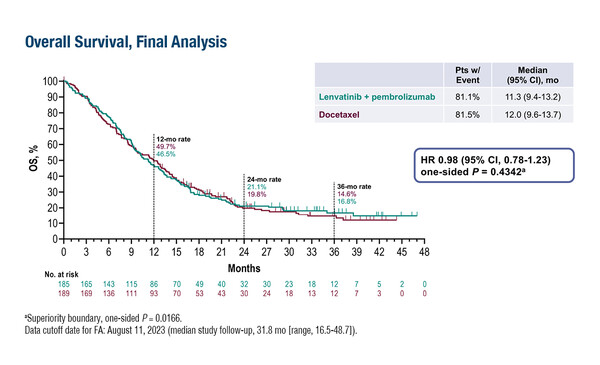
In the first study, the LEAP-006 phase III trial, 748 patients with previously untreated metastatic non-squamous NSCLC were randomised (1:1) to lenvatinib or placebo on top of pembrolizumab plus standard chemotherapy, with a comparable median overall survival (OS) between arms (21.8 months versus 22.1 months, respectively; hazard ratio [HR] 1.05; 95% confidence interval [CI] 0.88–1.26; p=0.708) (Abstract 64O). Similar results were reported from the LEAP-008 phase III trial involving 422 patients with NSCLC who progressed after immune checkpoint inhibition (ICI) plus chemotherapy. Patients were randomised (4:4:1) to lenvatinib plus pembrolizumab, docetaxel or lenvatinib, and there was no difference in median OS between the lenvatinib–pembrolizumab group and the docetaxel group (11.3 months versus 12.0 months, respectively; HR 0.98; 95% CI 0.78–1.23; p=0.434) (Abstract 65O). No unexpected adverse events were observed in either trial.
“Most patients with NSCLC do not have targetable alterations and while our current standard first-line treatment of ICI plus chemotherapy has led to some improvements, it may not be sufficient for some patients, either because of a short-lived response or no response,” comments Prof. Jarushka Naidoo from Beaumont Hospital, RCSI University of Health Sciences (Beaumont RCSI Cancer Centre), Dublin, Ireland. In the second-line setting, Naidoo also comments on the results from the LEAP-008 trial that add to the growing number of disappointing trials of strategies attempting to overcome immunotherapy resistance in NSCLC, including the SAPPHIRE phase III trial presented at the ESMO Congress 2023. “Despite the huge unmet need, innovating in this space is proving difficult. To make progress, we need to assess mechanisms of immunotherapy resistance more carefully and not consider patients with primary or acquired resistance as a single population.”
Lenvatinib plus pembrolizumab is approved for the first-line treatment of renal-cell carcinoma and previously treated endometrial cancer, and has shown encouraging activity in treatment-naïve or previously treated melanoma (J Clin Oncol. 2023;41:75–85). Lenvatinib inhibits VEGFR, FGFR, PDGFR, RET and KIT (Vasc Cell. 2014:6:18) and Naidoo speculates that either lenvatinib specifically is not effective at engaging these pathways for therapeutic benefit in NSCLC or that this class of agents may not be the preferred choice to engage these pathways in patients with lung cancer. The reason for the lack of improvement in NSCLC may also lie in its immunogenicity: “It was somewhat of a surprise when patients with NSCLC responded in the first immunotherapy trials as lung cancer was not thought of as classically immunogenic. The results from the LEAP trials provide more evidence that not all immunogenic tumour types behave in the same way,” she concludes.
Abstracts discussed:
Herbst RS, et al. Lenvatinib plus pembrolizumab, pemetrexed, and a platinum (len + pembro + chemo) as first-line therapy for metastatic nonsquamous non-small cell lung cancer (NSCLC): phase 3 LEAP-006 study. ESMO Immuno-Oncology Congress 2023, Abstract 64O
Proffered Paper Session 1, 06.12.2023, h. 14:15 – 15:45, Room B
Leighl N, et al. Phase 3 LEAP-008 study of lenvatinib plus pembrolizumab versus docetaxel for metastatic non-small cell lung cancer (NSCLC) that progressed on a PD-(L)1 inhibitor and platinum-containing chemotherapy. ESMO Immuno-Oncology Congress 2023, Abstract 65O
Proffered Paper Session 1, 06.12.2023, h. 14:15 – 15:45, Room B