Subgroup analyses from the ADRIATIC study support previous practice-changing findings
After a first interim analysis of the ADRIATIC study, significant improvements in overall survival (OS) and progression-free survival (PFS) were reported with durvalumab versus placebo consolidation in patients with limited-stage small cell lung cancer (LS-SCLC) without progression after concurrent chemoradiotherapy (J Clin Oncol. 2024;42(Suppl):LBA5). Newly-presented data from a subgroup analysis support the use of immunotherapy, irrespective of prior concurrent chemoradiotherapy and use of prophylactic cranial irradiation (PCI; LBA81).
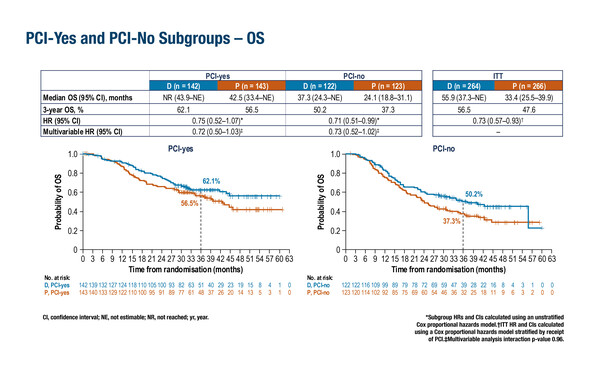
At the ESMO Congress 2024 (Barcelona, 13–17 September), subgroup analysis of patients who had received PCI (‘PCI-yes’; n=285) revealed that median OS was not reached (NR) in the durvalumab arm versus 42.5 months in the placebo arm (hazard ratio [HR] 0.75), while for patients who had not received PCI (‘PCI-no’; n=245), respective median OS was 37.3 months versus 24.1 months (HR 0.71). PFS in these subgroups was longer for patients in the durvalumab arm than the placebo arm, regardless of whether they received PCI (28.2 months versus 13.0 months, respectively; HR 0.73) or not (9.1 months versus 7.4 months, respectively; HR 0.80). Similarly, subgroup analysis of efficacy by twice-daily (BID; n=148) and once-daily (QD; n=382) radiotherapy administration showed that OS and PFS favoured the durvalumab arm compared with the placebo arm, regardless of radiotherapy schedule, although efficacy was longer with BID radiotherapy. Likewise, subgroup analysis of efficacy by choice of platinum, either carboplatin (n=179) or cisplatin (n=351) chemotherapy, demonstrated superiority of the durvalumab regimen over placebo in terms of OS and PFS with either regimen.
Higher rates of grade 3–4 treatment-emergent adverse events (TEAEs) were reported for both durvalumab and placebo in the respective ‘PCI-yes’ (28.4% and 29.6%) versus the ‘PCI-no’ subgroups (19.8% and 17.9%), and also in the carboplatin (31.5% and 31.8%) versus cisplatin subgroups (20.8% and 20.3%), while rates of TEAEs leading to durvalumab discontinuation were similar across subgroups. No meaningful differences in safety were found between BID and QD subgroups.
Dr Stephen Liu from Georgetown University, Washington, DC, USA, notes that in the present analysis, some small differences were observed between subgroups, for example, patients who received PCI had longer absolute OS and PFS than those who did not, regardless of treatment arm. In addition, BID radiotherapy was associated with improved absolute survival compared with QD radiotherapy. However, Liu cautions that, “These comparisons are problematic as PCI would only be considered in more robust patients with better performance status, and the same is true for BID versus QD radiotherapy. There are many potential confounders to consider. The groups that did better had more favourable baseline characteristics, which are not balanced.” Notably, results from multivariable analyses adjusting for differences in patient characteristics and in PCI and concurrent chemoradiotherapy received, showed no significant interactions.
“The durvalumab regimen demonstrated an impressive tolerability profile,” continues Liu, “and the toxicity data are reassuring given that patients are receiving treatment after radiation.”
Liu considers the data critical for the future treatment of LS-SCLC. “The intention of standard chemoradiotherapy in this setting is to provide a cure, but in reality this is an aspirational goal. We can see from the control arm that most people are not cured. However, these data make the possibility of cure a reality for more patients with LS-SCLC, so this is immediately practice-changing and every patient deserves to benefit,” he concludes.
Programme details
Senan S, et al. Durvalumab (D) as consolidation therapy in limited-stage SCLC (LS-SCLC): Outcomes by prior concurrent chemoradiotherapy (cCRT) regimen and prophylactic cranial irradiation (PCI) use in the ADRIATIC trial. ESMO Congress 2024, LBA81
Proffered Paper Session – Non-metastatic NSCLC, 13.09.2024, h. 14:00 – 15:30, Bilbao Auditorium – Hall 2