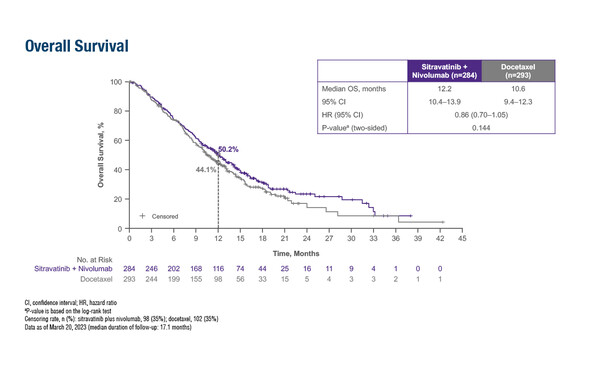
The SAPPHIRE trial failed to meet its primary endpoint compared with chemotherapy
According to results from the phase III SAPPHIRE trial presented at the ESMO Congress 2023 (Madrid, 20–24 October), there was no difference in the overall survival (OS) of patients who received sitravatinib with nivolumab compared with docetaxel as second- or third-line treatment for advanced non-squamous non-small-cell lung cancer (NSCLC) following progression on or after immune checkpoint inhibition (ICI) and chemotherapy (LBA63). Across 577 patients, median OS was 12.2 months with sitravatinib plus nivolumab versus 10.6 months with docetaxel (hazard ratio [HR] 0.86; 95% confidence interval [CI] 0.70–1.05; p=0.144). In addition, no significant differences between sitravatinib plus nivolumab and docetaxel were seen in median progression-free survival (4.4 months versus 5.4 months, respectively; HR 1.08; 95% CI 0.89–1.32; p=0.452) or objective response rate (16% versus 17%, respectively; p=0.597). Clinical benefit rate was 76% in the sitravatinib plus nivolumab arm, which was significantly higher than in the docetaxel arm (65%; p=0.004).
Safety profiles were consistent with prior reports for each regimen. The most common any grade treatment-related adverse events were diarrhoea (56%), nausea (31.3%) and decreased appetite (29%) with the combination of sitravatinib plus nivolumab, and diarrhoea (36%), fatigue (36%), nausea and decreased neutrophil count (32% each) with docetaxel.
The shift towards using ICIs as first-line treatment for advanced NSCLC has left an unmet need for effective subsequent treatment options as discussed in an editorial by Prof. Noemi Reguart. Based on phase II findings, it was thought that immunotherapy rechallenge with the addition of the immune-stimulating effects of sitravatinib may help reverse resistance (J Thorac Oncol. 2023;18:907–921); however, results from SAPPHIRE indicate no improvements over standard chemotherapy. Providing further validation, the phase III SAFFRON-301 trial – due to complete in 2024 – is evaluating sitravatinib in combination with the PD-1 inhibitor, tislelizumab, in patients with locally advanced/metastatic NSCLC who experienced disease progression after ICI and chemotherapy (NCT04921358).
Abstract discussed:
Borghaei H, et al. SAPPHIRE: Phase 3 study of sitravatinib plus nivolumab versus docetaxel in patients with previously treated advanced non-squamous non-small cell lung cancer (NSCLC). ESMO Congress 2023, LBA63
Proffered Paper Session – NSCLC, metastatic, 20.10.2023, h. 16:00 – 17:30, Barcelona Auditorium – Hall 9