Studies reveal promising evidence for the more precise tailoring of immunotherapy and the role of AI in improving response evaluation
The approval of nivolumab plus ipilimumab, based on the CheckMate 743 trial, changed the face of treatment for unresectable malignant pleural mesothelioma (PM). At the ESMO Congress 2024 (Barcelona, 13–17 September), a molecular analysis of data from the CheckMate 743 trial reported distinctly different outcomes with immunotherapy depending on tumour mutations in BAP1/CDKN2A/CDKN2B genes (Abstract 1921P). Among 251 tumour samples, immunotherapy was associated with better median overall survival (OS) than chemotherapy in patients with triple mutations and epithelioid histology (14 months versus 10.9 months) and in patients with double mutations and PD-L1-positive disease, irrespective of histology (15.8 months versus 9.4 months). Tumours with mutations in BAP1 only were associated with the best prognosis on either treatment and also had the most inflamed gene signature profile, with significantly higher levels of type-I interferon and T-cell trafficking. In patients with tumours lacking BAP1/CDKN2A/CDKN2B mutations, there was no difference in OS between immunotherapy and chemotherapy. The results warrant further study to clarify the role of BAP1/CDKN2A/CDKN2B mutations as biomarkers for tailoring immunotherapy in the treatment of PM.
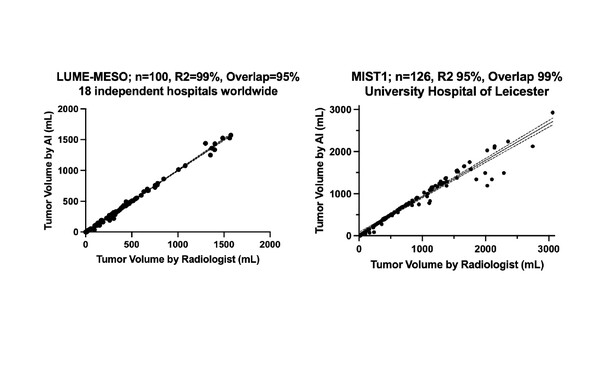
Another study reported on the potential for artificial intelligence (AI) to improve response evaluation, based on data from computed tomography (CT) scans from patients with PM in 27 countries (Abstract 1910MO). Over a combination of seven clinical trials (3,532 CT scans), the AI-derived total tumour volume (TTV) Automated Response evaluation to Treatment In MESothelioma (ARTIMES) outperformed mRECIST criteria, with concordance indices of 0.76 and 0.71, respectively. In addition, ARTIMES identified disease progression a median 7 weeks earlier than mRECIST (p<0.0001). Finally, a significant prognostic value of the AI-derived baseline TTV was observed (651 CT scans; p<0.0001). This model, which could provide accurate, cost-effective response monitoring, is undergoing prospective validation and clinical implementation.
AI & Digital Oncology: Resources in one place
Looking for further insights into how artificial intelligence and digital tools are impacting oncology? The ESMO AI & Digital Oncology Hub brings together expert perspectives, research updates, and thought leadership from across oncology.
It is a space where you can stay informed, discover resources, and follow the conversation on digital innovation in cancer research and treatment.
To further explore the transformative potential of AI in oncology, the very first ESMO AI and Digital Oncology Congress 2025, taking place from 12 to 14 November, will provide a dedicated platform focused on the latest advances in AI and digital technologies in cancer care.