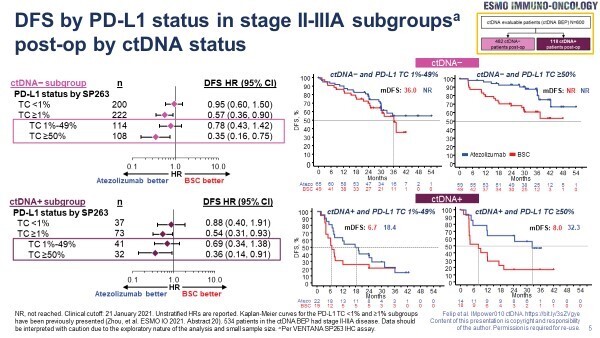
However, improved outcomes are reported in PD-L1-positive patients only irrespective of post-operative and post-chemotherapy ctDNA status, calling into question its predictive value
An exploratory analysis of the IMpower010 study, involving 600 patients with resected non-small-cell lung cancer (NSCLC), confirms that circulating tumour (ct)DNA has prognostic utility for adjuvant atezolizumab but that its predictive value is still uncertain (Abstract 1O). Data presented at ESMO Immuno-Oncology Congress 2022 (Geneva, 7–9 December) show that atezolizumab improved disease-free survival (DFS) compared with best supportive care (BSC) across PD-L1-positive subgroups (tumour cell [TC] ≥1%, 1–49% and ≥50%) – but not in the PD-L1-negative (TC <1%) subgroup – irrespective of post-operative ctDNA status.
ctDNA clearance following chemotherapy, but before atezolizumab, was achieved in 62% (64/103) of patients with post-operative ctDNA positivity, and was linked to improved DFS. However, the DFS benefit of atezolizumab over BSC was apparent both in patients with post-chemotherapy ctDNA clearance (31.3 months versus 13.3 months; hazard ratio [HR] 0.7; 95% confidence interval [CI] 0.37–1.34) and in patients without post-chemotherapy ctDNA clearance (4.2 months versus 3.9 months; HR 0.67; 95% CI 0.34–1.32). In a longitudinal assessment of the 39 patients who were ctDNA-positive following chemotherapy, ctDNA levels were maintained by atezolizumab over time, but increased approximately 10-fold with BSC. Among patients who were ctDNA-negative post-chemotherapy, atezolizumab was associated with a longer median time to convert to ctDNA positivity versus BSC (not reached versus 4.7 months; HR 0.6; 95% CI 0.31–1.17) and an improved DFS (31.3 months versus 13.3 months; HR 0.70; 95% CI 0.37–1.34).
This follow-up analysis endorses previous data presented by Zhou et al. at the ESMO Immuno-Oncology Congress 2021 suggesting that post-operative ctDNA positivity is a strongly prognostic biomarker for DFS in early-stage NSCLC and provides important new information regarding post-chemotherapy ctDNA. “The results validate ctDNA as a promising tool for sensitive monitoring of minimal residual disease (MRD) in the curative setting and show the potential to individualise escalation and de-escalation of treatment strategies through the peri-operative disease setting,” says Prof. Noemí Reguart from Hospital Clinic Barcelona, Spain, commenting on the data presented. “However, there is not yet enough evidence to consider MRD alone as a predictive biomarker for atezolizumab treatment selection, given that adjuvant atezolizumab improved DFS compared with BSC regardless of post-operative and post-chemotherapy ctDNA status. This calls into question the sensitivity and negative predictive value of the ctDNA technique to identify MRD.”
These results do not discount the future potential for ctDNA use in both the pre- and post-operative settings. “We know that tumour PD-L1 expression is not an ideal biomarker and that there is a need to further enrich the patient population at higher risk of recurrence to help optimise treatment decisions and reduce financial costs,” explains Prof. Reguart. “Unfortunately, all the data on the role of MRD and ctDNA so far are based on exploratory or retrospective analyses. We need more prospective, randomised studies of treatment individualisation and to integrate these data with PD-L1 tumour expression,” she says.
Abstract discussed:
Felip E, et al. IMpower010: ctDNA status in patients (pts) with resected NSCLC who received adjuvant chemotherapy (chemo) followed by atezolizumab (atezo) or best supportive care (BSC). ESMO Immuno-Oncology Congress 2022, Abstract 1O
Proffered Paper Session 07.12.2022, h. 14:05 – 15:40, Room B. Also watch the session on the Congress virtual platform