Results from an exploratory analysis of the IMpower010 trial confirm that there is a correlation between tumour-cell PD-L1 expression and disease-free survival
The IMpower010 trial reported earlier this year that atezolizumab improved disease-free survival (DFS) compared with best supportive care (BSC) in patients with stage II–IIIA resected non-small cell lung cancer (NSCLC) (Lancet. 2021;398(10308):1344–1357). Patients were stratified by SP142-determined PD-L1 status.
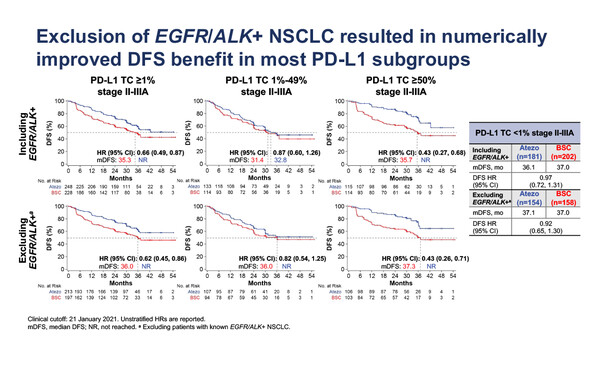
In a presentation at the ESMO Immuno-Oncology Congress 2021 today, outcomes according to SP263-determined PD-L1 subgroups among 859 evaluable patients with stage II–IIIA disease showed DFS improvements with atezolizumab versus BSC across all subgroups with PD-L1 tumour cell (TC) ≥1%: PD-L1 TC ≥1% (hazard ratio [HR] 0.66; 95% confidence interval [CI] 0.49–0.87), PD-L1 TC 1–49% (HR 0.87; 95% CI 0.60–1.26), PD-L1 TC ≥50% (HR 0.43; 95% CI 0.27–0.68) (Abstract 2O). There was no DFS difference between atezolizumab and BSC in patients with PD-L1 TC <1% (HR 0.97; 95% CI 0.72–1.31). When patients with EGFR/ALK-positive disease were excluded, numerical improvements in DFS HRs were seen in all PD-L1 subgroups (PD-L1 TC <1%, HR 0.92; PD-L1 TC ≥1%, HR 0.62; PD-L1 TC 1–49%, HR 0.82), except PD-L1 TC ≥50%, in which the HR was the same, 0.43.
The findings demonstrate that the use of SP263 to assign patients to the different PD-L1 expression subgroups compares very well with the original SP142-determined stratification, and this is extremely important for the interpretation of the results.
Prof. Martin Reck, Lung Clinic Grosshansdorf, Germany, and an investigator on the study, comments: “The analysis provides valuable information about the patients who are unlikely to respond to atezolizumab in this context. It confirms earlier signals that there is a correlation between tumour cell PD-L1 expression and DFS and that oncogenic alterations are probably an unfavourable marker for atezolizumab use in this population,” he explains.
The analysis also assessed the biomarker potential of post-surgery circulating tumour (ct)DNA status. Among 534 ctDNA-evaluable patients, ctDNA positivity increased with disease stage, being reported in 14% of patients with stage II NSCLC and 29% with stage IIIA disease. Across disease stages, ctDNA positivity was associated with a worse prognosis; among patients receiving BSC, the median DFS was 7.9 months in ctDNA-positive patients and it was not reached (NR) in ctDNA-negative patients. Atezolizumab improved DFS compared with BSC in both ctDNA-negative and ctDNA-positive patients, the benefit being greater in patients with PD-L1 TC ≥1%. Among ctDNA-negative patients, median DFS times with atezolizumab and BSC were NR (HR 0.95; 95% CI 0.60–1.50) for PD-L1 TC <1% and NR versus 37.3 months (HR 0.57; 95% CI 0.36–0.90) for PD-L1 TC ≥1%. Among ctDNA-positive patients, median DFS times with atezolizumab and BSC were 5.1 months versus 8.0 months (HR 0.88; 95% CI 0.40–1.91) for PD-L1 TC <1% and 21.8 versus 7.2 months (HR 0.54; 95% CI 0.31–0.93) for PD-L1 TC ≥1%.
“The ctDNA findings are very exciting and are a clear milestone for further research,” says Reck. “The presence of ctDNA after resection is a very clear poor prognostic marker and we know that it also correlates with other unfavourable prognostic factors, such as poor performance status. In terms of efficacy, the benefit of atezolizumab over BSC is seen in both ctDNA-positive and -negative patients but is greater in patients with PD-L1 expression ≥1%,” he continues. “While ctDNA is not yet ready for use in clinical practice, it should be incorporated into future trials and possibly used as a stratification factor for novel perioperative therapies,” Reck concludes.
Zhou C, et al. IMpower010: biomarkers of disease-free survival (DFS) in a Phase 3 study of atezolizumab (atezo) vs best supportive care (BSC) after adjuvant chemotherapy in stage IB-IIIA NSCLC. ESMO Immuno-Oncology Congress 2021, Abstract 2O
Proffered Paper Session 1, 09.12.2021, h. 09:00 – 10:30, Room A