The phase II part of the ROCSAN trial failed to meet its primary endpoint but there may be potential for benefit based on efficacy and safety data with the combination
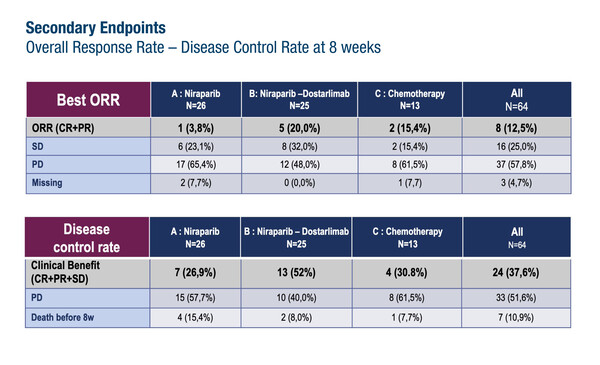
The two-step ROCSAN trial was designed to evaluate the effects of PARP inhibition with or without PD-1 inhibition in the treatment of metastatic or recurrent endometrial/ovarian carcinosarcoma after at least one line of platinum-based chemotherapy, a disease associated with poor prognosis and limited treatment options. According to results from the phase II part of the trial, presented at the ESMO Gynaecological Cancers Congress 2024 (Florence, 20–22 June), the primary endpoint of 16-week response rate of >20% was not met in patients randomised to niraparib (3.8%) or to niraparib plus dostarlimab (12.0%), with a rate of 15.4% in patients who received chemotherapy (Abstract 34O). The study included 64 patients (median age, 70 years), of whom 82.8% had endometrial carcinosarcoma and 34.4% had previously received two or three lines of chemotherapy.
Other efficacy findings revealed overall (complete plus partial) response rates of 3.8%, 20.0% and 15.4%, for niraparib, niraparib plus dostarlimab and chemotherapy, respectively, and corresponding 8-week disease control rates of 26.9%, 52.0% and 30.8%.
At a median follow-up of 11.2 months, the median progression-free survival was 2.0 months with niraparib, 2.7 months with niraparib plus dostarlimab and 1.9 months with chemotherapy. Corresponding overall survival times were 6.7 months, 6.5 months and 4.5 months, respectively. At least one adverse event of grade 3 or more related to study treatment was reported by 61.5% of patients in the niraparib arm, by 44.0% for niraparib and by 8.0% for dostarlimab in the niraparib plus dostarlimab arm, and by 0–30.8% of patients in the chemotherapy arm (depending on the chemotherapy regimen).
An amendment has been submitted to continue the ROCSAN trial as an exploratory extended phase II single-arm study with the niraparib plus dostarlimab combination in immunotherapy-naïve patients with carcinosarcoma.
Abstract discussed:
Ray-Coquard IL, et al. ROCSAN: A multicentric randomized phase II/III evaluating dostarlimab in combination with niraparib versus niraparib alone compared to chemotherapy in the treatment of endometrial/ovarian carcinosarcoma after at least one line of platinum-based chemotherapy, preliminary results. ESMO Gynaecological Cancers Congress 2024, Abstract 34O
Proffered Paper Session, 20.06.2024, h. 14:45 – 16:15, Auditorium