New survival data from the MIRASOL trial support the use of this antibody-drug conjugate as a preferred standard of care for patients with high folate receptor alpha tumour expression
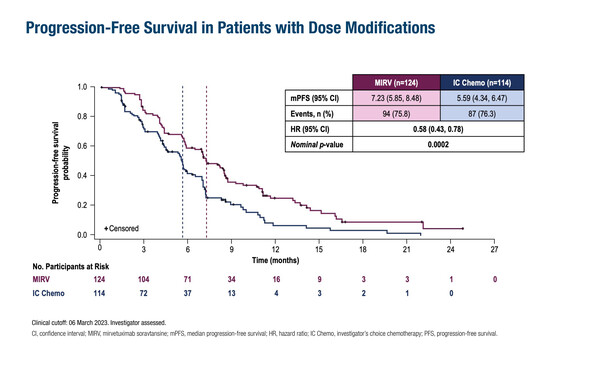
As presented at the ESMO Gynaecological Cancers Congress 2024 (Florence, 20–22 June), a post-hoc analysis of the phase III MIRASOL (GOG 3045/ENGOT-ov55) trial in patients with high folate receptor alpha (FRalpha), platinum-resistant ovarian cancer revealed that even those who required treatment dose modifications achieved clinically meaningful improvements with the antibody–drug conjugate (ADC) mirvetuximab soravtansine (MIRV) compared with investigator’s choice of chemotherapy (Abstract 44O). Similar proportions of patients required dose modifications in each arm – 124 (55%) patients with MIRV and 114 (50%) with standard chemotherapy – and in these patients, progression-free survival was significantly longer with MIRV (hazard ratio [HR] 0.58; 95% confidence interval [CI] 0.43–0.78; nominal p=0.0002). MIRV also showed benefits over chemotherapy in overall survival (HR 0.45; 95% CI 0.30–0.68; nominal p=0.0001) and objective response rate (60% versus 26%, respectively).
Rates of grade ≥3 treatment-emergent adverse events (TEAEs) and serious AEs were lower with MIRV (53% and 24%, respectively) versus standard chemotherapy (72% and 39%, respectively). TEAE-related treatment discontinuations occurred in 10% of patients receiving MIRV and 22% of patients receiving standard chemotherapy.
“The results of this analysis strengthen the unprecedented primary findings from the trial published last year (N Engl J Med. 2023;389:2162–2174),” comments Dr Robert L. Coleman from Texas Oncology, TX, USA. “Modifying the dose to keep within the therapeutic index is a balancing act and has the potential to lead to delivery of ineffective treatment. In the MIRASOL trial, although dose modifications were required by at least half of the patients in each treatment arm, they did not negatively impact efficacy and MIRV sustained its survival benefits over standard chemotherapy,” he says. Coleman continues: “We know that some issues raised by the MIRASOL trial still need to be investigated further, such as its lack of an ethnically diverse population. However, advantages in overall survival have been extremely difficult to show in ovarian cancer trials, because of the extent of crossover therapies available, and this makes the results seen with MIRV in the MIRASOL trial all the more impressive.”
Coleman finishes by noting that: “In gynaecological cancers, tisotumab vedotin was the first ADC to be approved for patients with cervical cancer; MIRV is the first ADC approved for ovarian cancer patients. The success of these two agents has infused tremendous enthusiasm for the development of other ADCs, broadening tissue targets and cytotoxic payloads. The developmental landscape holds new promise to improve the outcomes of patients with these difficult-to-treat types of gynaecological cancer and that can only be good news.”
The expanding role of ADCs in ovarian cancers will be discussed in an educational session at the Congress on Saturday.
Don't miss
Banerjee S, et al. Safety and efficacy results in patients who received dose modifications in the phase 3 MIRASOL (GOG 3045/ENGOT-ov55) trial of mirvetuximab soravtansine vs investigator’s choice chemotherapy (ICC) in platinum-resistant ovarian cancer (PROC) with high folate receptor-alpha expression. ESMO Gynaecological Cancers Congress 2024, Abstract 44O
Proffered Paper Session, 20.06.2024, h. 14:45 – 16:15, Auditorium
Expanding our therapeutic armamentarium: ADCs in the ovarian cancer field
Educational Session, 22.06.2024, h. 10:20 – 11:50, Auditorium