Positive results are reported from the FZOCUS-1 and ICON8B studies
Survival data from two phase III trials presented at the ESMO Congress 2025 (Berlin, 17–21 October) highlight possible shifts in the standard of care for patients with advanced ovarian cancer.
It is widely established that maintenance monotherapy with PARP inhibitors improves progression-free survival (PFS) in patients with ovarian cancer who respond to first-line platinum-based chemotherapy, while the combination of PARP inhibitor plus bevacizumab provides a clinically meaningful improvement in overall survival (OS) in the first-line treatment of those with homologous recombination deficient (HRD)-positive ovarian cancer. The randomised placebo-controlled FZOCUS-1 trial explored the potential added benefit of combining the PARP inhibitor fuzuloparib with the anti-angiogenic agent apatinib in Asian patients newly diagnosed with ovarian cancer who had responded to platinum-based chemotherapy (Abstract 1063O). In the overall population, no added benefit was seen with combination fuzuloparib plus apatinib (n=269) over fuzuloparib monotherapy (n=269; median PFS [mPFS] 26.9 months [95% confidence interval [CI] 20.3–36.6] versus 29.9 months [95% CI 22.1–36.1], respectively).
“Combining fuzuloparib and apatinib may offer a benefit specifically in patients with homologous recombination proficient (HRP) tumours; in the study, a trend toward improved PFS was observed with combination therapy compared to monotherapy in this subgroup (mPFS 16.6 months [95% CI 10.2–22.1] versus 11.0 months [95% CI 6.5–16.6], respectively),” says Prof. Isabelle Ray-Coquard from the Centre Léon Bérard, Lyon, France, commenting on the results.
“However, further studies are needed to confirm the global applicability, as this trial enrolled a fully Asian population and involved agents not widely used in current international treatment practice. Also, consideration must be given to any added value of fuzuloparib plus apatinib in this population, given the findings for bevacizumab monotherapy in the PAOLA-1 (Ann Oncol. 2023;34:681–692) and GOG-0218 (J Clin Oncol. 2019;37:2317–2328) studies.”
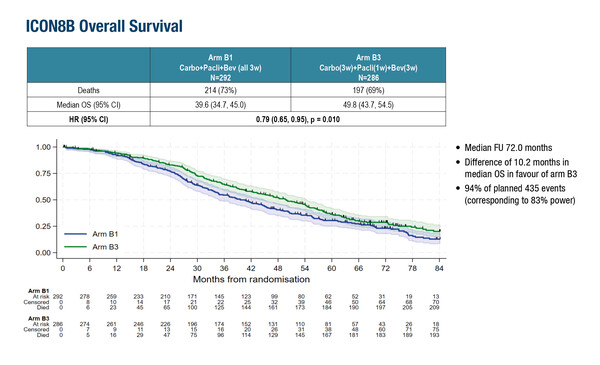
In the high-risk stage III–IV epithelial ovarian cancer setting, potentially practice-changing findings were presented from the ICON8B trial where enrolled patients received first-line combination therapy with paclitaxel, carboplatin and the anti-angiogenic agent bevacizumab (n=579). Previously, initial results showed that bevacizumab added to weekly paclitaxel compared with 3-weekly dosing resulted in a significantly longer mPFS (22.2 months versus 16.7 months, respectively; hazard ratio [HR] 0.75; 95% CI 0.62–0.90; p=0.002) (Int J Gynecol Cancer. 2023;33(Suppl3):A424). The final analysis now demonstrates that weekly paclitaxel improves median overall survival (mOS) by 10.2 months over the 3-weekly regimen (mOS 49.8 months [95% CI 43.7–54.5] versus 39.6 months [95% CI 34.7–45.0], respectively; HR 0.79; 95% CI 0.65–0.95; p=0.010) again when bevacizumab is added on top of standard chemotherapy (Abstract 1064O).
“This improvement is clinically meaningful. Weekly paclitaxel also has advantages such as less alopecia, and it will be interesting to compare quality of life outcomes between the two regimens,” says Ray-Coquard. However, she cautions that the results must be interpreted in context, noting several limitations including the lack of BRCA mutation stratification and the fact that the trial did not reflect current standards of care, such as the use of PARP inhibitors in patients with HRD tumours or hyperthermic intraperitoneal chemotherapy during debulking surgery, both of which are now known to improve both PFS and OS.
Programme details:
Li N, et al. Fuzuloparib (FZPL) monotherapy or in combination with apatinib (APA) as first-line (1L) maintenance therapy in advanced ovarian cancer (OC): Final analysis of the FZOCUS-1 trial. ESMO Congress 2025 - Abstract 1063O
Clamp AR, et al. ICON8B: GCIG Phase III randomised trial comparing first-line weekly dose-dense chemotherapy + bevacizumab to three-weekly chemotherapy + bevacizumab in high-risk stage III-IV epithelial ovarian cancer (EOC): Final overall survival (OS) analysis. ESMO Congress 2025 - Abstract 1064O