The ENGOT-ov65/KEYNOTE-B96 trial reported small but clinically relevant progression-free survival increments and significantly improved overall survival in platinum-resistant ovarian cancer
The addition of pembrolizumab to weekly paclitaxel, with or without bevacizumab, increased progression-free survival (PFS) and overall survival (OS) in the phase III ENGOT-ov65/KEYNOTE-B96 trial of 643 patients with platinum-resistant ovarian cancer (PROC). “These data are very interesting and may change how we think about the use of immunotherapy in ovarian cancer, since this is the first time we have seen an OS signal with the addition of an immune checkpoint inhibitor in a non-histologically selected population,” highlights Dr Rebecca Kristeleit from Guy’s and St Thomas’ NHS Foundation Trust and King's College London, UK, commenting on the findings presented in today's Presidential Symposium at the ESMO Congress 2025 (Berlin, 17–21 October).
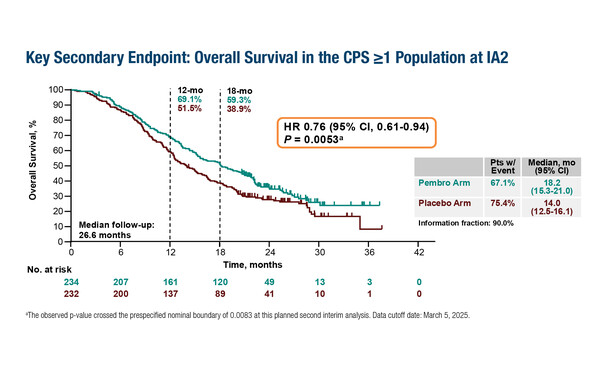
In the first interim analysis, at a median follow-up of 15.6 months, the primary endpoint of PFS was 8.3 months in the pembrolizumab arm versus 6.4 months in the placebo arm in the non-selected intention-to-treat population (hazard ratio [HR] 0.70; 95% confidence interval [CI] 0.58–0.84; p<0.0001) and 8.3 months versus 7.2 months, respectively, in patients with PD-L1-positive tumours (PD-L1 combined positive score [CPS] ≥1) (HR 0.72; 95% CI 0.58–0.89; p=0.0014). In the second interim analysis, at a median follow up of 26.6 months, OS was significantly improved in the PD-L1 CPS ≥1 population (18.2 months versus 14.0 months; HR 0.76; 95% CI 0.61–0.94; p=0.0053) (LBA3). “The PFS increments are small, but clinically relevant in this patient population, and the significant improvement in OS, albeit with PD-L1 selection, suggests that tumour shrinkage is maintained. It will be interesting to see how the observed differences between the PD-L1-negative and PD-L1-positive (suggesting an immune evasive phenotype) populations evolve over the longer term. In particular, identifying whether the OS improvement in PD-L1-positive patients might derive from a positive impact of immunotherapy on the efficacy of subsequent therapies due to changes in the immune microenvironment,” notes Kristeleit.
PROC remains an area of high unmet need. Several trials have previously demonstrated the benefits of bevacizumab and pembrolizumab in this setting and provided a strong rationale for the ENGOT-ov65/KEYNOTE-B96 trial. A doubling of PFS was reported with the addition of bevacizumab to weekly paclitaxel in the phase III AURELIA study (J Clin Oncol. 2014;32:1302–1308), and a response rate of 51.4% was achieved with pembrolizumab plus weekly paclitaxel in a subsequent phase II study (Int J Gynecol Cancer. 2018;28(Suppl 2):IGCS8-1482). A third study (LEAP-005) reported a response rate of 35% with a chemotherapy-free regimen of pembrolizumab and lenvatinib in heavily pre-treated patients with ovarian cancer (Gynecol Oncol. 2024;186:182–190).
In ENGOT-ov65/KEYNOTE-B96, 86.0% had high-grade serous histology and 7.8% had clear-cell histology. Around three-quarters of patients (73.3%) had bevacizumab use at baseline. Effects on OS at the second interim analysis in the PD-L1 CPS ≥1 population appeared meaningful regardless of bevacizumab use (HR 0.75; 95% CI 0.58–0.97) or not (HR 0.75; 95% CI 0.51–1.11). “Insights may also be provided by investigating the impact on clinical activity of various factors including tumour histology, the pattern of recurrence (including whether any debulking surgery had been performed), subsequent therapy and markers that might predict sensitivity to immunotherapy, such as mismatch repair, tumour mutational burden status and levels of tumour infiltrating lymphocytes,” she says.
Kristeleit notes that this regimen may have potential in patients with PROC who have received more than 2 prior lines of therapy, and possibly in other groups with rare histological subtypes including non-epithelial ovarian cancer subtypes such as granulosa and Sertoli cell, where chemotherapy alone is not particularly effective. However, accessibility to the three-drug regimen may be limited: “Some regulatory authorities may not approve this regimen for an unselected population for what might be considered quite a small gain in clinical benefit where treatment choice will depend to a large extent on toxicity.” In the study, the safety profile of pembrolizumab plus weekly paclitaxel, with or without bevacizumab, was described as manageable, although grade ≥3 treatment-related adverse events were reported in 67.5% versus 55.3% of participants, respectively.
Additional hope for the treatment of PROC comes from the use of antibody–drug conjugates (ADCs), which are emerging as promising therapies in ovarian and other gynaecological cancers (Gynecol Oncol. 2025;195:180–191; Oncogene. 2025;44:2343–2356). “ADCs may be another class of agents that could be combined with pembrolizumab and bevacizumab in PROC given the significant clinical benefit observed, including improved OS, with ADCs in this setting (N Engl J Med. 2023;389:2162–2174),” suggests Kristeleit, “Although, ADCs may be more widely applicable in PROC, for example, if patients are not fit enough to tolerate the triple combination.”
Programme details
Colombo N, et al. Pembrolizumab vs placebo plus weekly paclitaxel ± bevacizumab in platinum-resistant recurrent ovarian cancer: results from the randomized double-blind phase 3 ENGOT-ov65/KEYNOTE-B96 study. ESMO Congress 2025 - LBA3