High expression of specific immune-oncology proteins may have utility in identifying high-risk patients to inform future trial design
Previously, the phase III ANITA trial showed that the addition of atezolizumab to platinum-based chemotherapy followed by niraparib maintenance did not improve progression-free survival (PFS) compared with standard therapy in patients with late-relapsing recurrent ovarian cancer (J Clin Oncol. 2024;42:4294–4304). Using multiplex cytokine profiling analysis to explore baseline circulating immune-oncology proteins in the ANITA trial participants, several immune and angiogenic markers were identified as potential predictive or prognostic biomarkers related to worse outcomes, according to a presentation at the ESMO Congress 2025 (Berlin, 17–21 October) (LBA41).
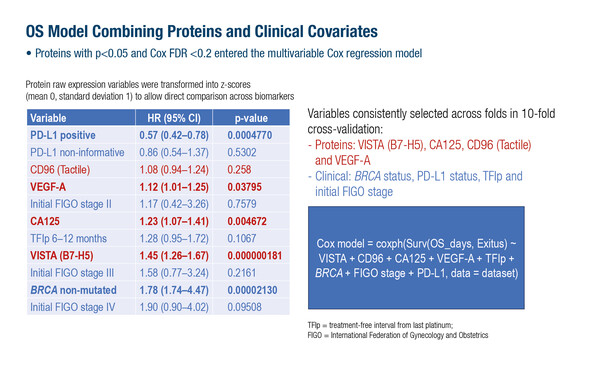
Multiplex profiling enables the simultaneous assessment of numerous circulating proteins in a high-throughput process using a small sample of blood. In the study, baseline plasma samples were obtained from 332 patients and analysed using a ProcartaPlex™ immunoassay; this is a customisable assay which enables the detection and quantitation of up to 80 protein targets from a 6.3–50 µL sample. Of 74 proteins related to immune regulation, angiogenesis and inflammation, univariate analyses identified VISTA (B7-H5), CD31 (PECAM-1), uPAR and VEGF-A levels as being significantly associated with worse PFS and overall survival (OS), and LOX-1 with worse OS. In a multivariable OS model that combined proteins and clinical variables, VISTA (B7-H5), CA125, VEGF-A and CD96 (Tactile) remained significant, enabling risk stratification for OS, with the strongest prediction at 6 months. Clinical variables associated with worse OS outcome were BRCA status, PD-L1 status, treatment-free interval from last platinum treatment and initial International Federation of Gynecology and Obstetrics stage. The worst PFS and OS were consistently associated with levels of VISTA (B7-H5) and VEGF-A. No significant interactions were found between protein expression and response to treatment in the ANITA trial, suggesting prognostic rather than predictive value.
Dr Joo Ern Ang from the Cambridge University Hospitals NHS Foundation Trust, UK, explained that VISTA and VEGF-A emerged in this study as the most relevant proteins associated with the poorest PFS and OS. “High expressions of these proteins were strongly and adversely prognostic, suggesting utility as stratifying factors when planning clinical trials. VISTA and VEGF-A expression, for instance, could be used to identify patients at highest risk, and may play in role in trial eligibility,” says Ang. However, he also acknowledges that, “If they were to be used for patient selection, for example, cutoff values for these protein levels would need to be established, and as the results are based on a post-hoc analysis of one clinical trial, additional validation of the data would also be required.”
VEGF has previously been identified as an adverse prognostic biomarker in ovarian cancer (Int J Gynecol Cancer. 2017 Sep;27(7):1325-1332), and VEGF inhibitors, such as bevacizumab, are associated with survival benefits in advanced disease in selected patients (Cancer Treat Rev. 2025;137:102945). “This analysis supports these observations and suggests that regulators of VEGF signalling and biology can impact on patient outcomes in this setting,” concludes Ang.
Programme details:
González-Martín A, et al. Baseline (BL) multiplex cytokine profiling identifies prognostic signatures in the Phase 3 ANITA (ENGOT-OV41/GEICO 69-O) trial in recurrent ovarian cancer (ROC). ESMO Congress 2025 - LBA41