While updated results from the DUO-O trial appear promising, they are not conclusive due to the study design
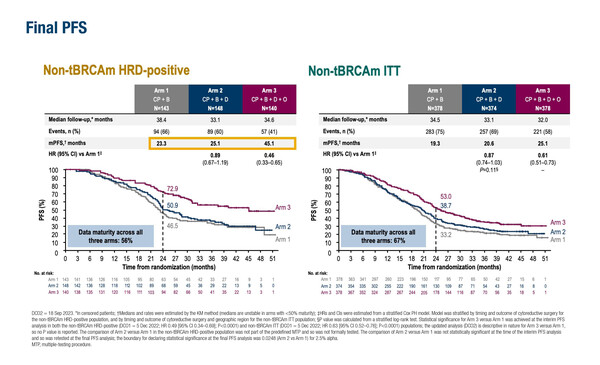
As presented at the ESMO Gynaecological Cancers Congress 2024 (Florence, 20–22 June), final progression-free survival (PFS) data from the phase III DUO-O trial in patients with newly diagnosed non-BRCA-mutated ovarian cancer confirm and extend previous results from an interim analysis (J Clin Oncol 2023;41(17 Suppl):LBA5506) (Abstract 43O). Median PFS was 23.3 months in the control arm of carboplatin/paclitaxel + bevacizumab followed by bevacizumab maintenance (CP + B) versus 45.1 months with carboplatin/paclitaxel + bevacizumab + durvalumab followed by durvalumab + olaparib + bevacizumab maintenance (CP + B + D + O) (hazard ratio [HR] 0.46; 95% confidence interval [CI] 0.33–0.65) in 283 patients with homologous recombination deficiency (HRD)-positive disease. In addition, benefits were sustained in the intention-to-treat (ITT) population of 756 patients, with PFS of 19.3 months in the control arm versus 25.1 months in the CP + B + D + O arm (HR 0.61; 95% CI 0.51–0.73). Results from another arm that received carboplatin/paclitaxel + bevacizumab + durvalumab followed by durvalumab + bevacizumab maintenance (CP + B + D) appeared similar to the control arm.
“The PFS of 45.1 months seen with the durvalumab plus olaparib combination is the longest reported to date in this population of patients,” says Prof. Domenica Lorusso from Humanitas San Pio X, Milan, Italy. “That said, unfortunately, the results of this trial have to be viewed as inconclusive as the control arm does not include a PARP inhibitor, which became the standard treatment for HRD-positive ovarian cancer while the DUO-O trial was in progress. This means that not only is the control regimen not the current standard of care for HRD-positive disease, but it is also impossible to ascertain the added contribution of immunotherapy to a PARP inhibitor in the investigational regimen.” She continues: “The lesson to researchers is that science is moving increasingly quickly and so, if we are to get reliable results from clinical studies, we need to use adaptive trial designs that can accommodate changes prompted by new evidence.”
Looking at the secondary endpoints, there was a trend towards an improvement in an interim analysis of overall survival in the CP + B + D + O arm versus the control arm in the HRD+ population (HR 0.84; 95% CI 0.51–1.37), but no benefit in the ITT population (HR 0.95; 95% CI 0.76–1.20). Updated safety data reflected findings from the initial analysis. Rates of adverse events leading to treatment discontinuation in the CP + B + D + O arm were 35% for the combined chemotherapy and maintenance phases and 27% for the maintenance phase, compared with corresponding rates of 22% and 15% in the control arm.
Lorusso notes that the use of five agents is a cause for concern: “If all the active agents are used in the first-line setting, we need to be sure to cure the patients at the first occurrence of disease otherwise, if the patient suffers disease recurrence, the clinician has few treatment options available. Even more importantly, toxicity is potentially problematic and the rates of discontinuation due to adverse events were high.” She concludes, “As clinicians, we need to be very confident that the incremental benefit of combining multiple therapies outweighs the issues.”
Abstract discussed
Trillsch F, et al. Durvalumab (D) + carboplatin/paclitaxel (CP) + bevacizumab (B) followed by D, B + olaparib (O) maintenance (mtx) for newly diagnosed advanced ovarian cancer (AOC) without a tumour BRCA1/BRCA2 mutation (non-tBRCAm): updated results from DUO-O. ESMO Gynaecological Cancers Congress 2024, Abstract 43O
Proffered Paper Session, 20.06.2024, h. 14:45 – 16:15, Auditorium