Ceralasertib, alone or in combination with PARP inhibition, showed signs of clinical efficacy in patients with relapsed ovarian and endometrial clear cell carcinomas (CCC), for whom effective treatment options are currently lacking
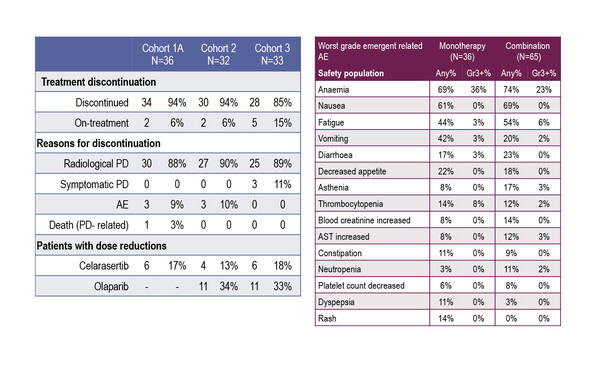
According to results from the phase II ENGOT/GYN1/NCRI ATARI trial presented at the ESMO Gynaecological Cancers Congress 2023 (Barcelona, 23–24 February), among 29 efficacy-evaluable patients with relapsed ovarian and endometrial CCC, monotherapy with the ataxia telangiectasia mutated and Rad3-related (ATR) inhibitor, ceralasertib, was associated with an objective response rate (ORR) of 14% (Abstract 34O). All tumours in this patient group presented with ARID1A protein loss (cohort 1A). The disease control rate ([DCR] ORR plus stable disease for at least 16 weeks) was 41%, with a median duration of response (DoR) of 24 weeks (interquartile range [IQR] 22.1–31.7) and a median progression-free survival (PFS) of 3.5 months (95% confidence interval [CI] 1.8–5.4) at a median (IQR) follow-up of 10.8 (6.9–20.9) months. Twenty-nine efficacy-evaluable patients with relapsed ovarian and endometrial CCCs but no ARID1A protein loss (cohort 2) received a combination of ceralasertib and olaparib, leading to an ORR of 14%, DCR of 38%, median (IQR) DoR of 8 (7.6–9.0) weeks and median PFS of 3.5 months (95% CI 2.9–5.3).
Of interest, in a third cohort of 29 efficacy-evaluable patients with tumours without CCC histology (carcinosarcomas, endometrioid and cervical cancers), the combination of ceralasertib plus olaparib resulted in an ORR of 21%, DCR of 52%, median (IQR) DoR of 41 (32.9–49.9) weeks and median PFS of 5.5 months (95% CI 1.8–8.3).
“These are very exciting results, showing that for the first time in these rare ovarian and endometrial cancers, we have the possibility to personalise treatment based on the tumour molecular profile,” says Prof. Isabelle Ray-Coquard from Centre Léon Bérard and GINECO, Lyon, France, who contributed to the study. “The response rates of 14% and a median PFS of close to 4 months in patients with CCC, with or without ARID1A protein loss, are very promising for patients with these types of tumours, which have generally no sensitivity to chemotherapy or other targeted treatments,” she observes. Regarding the efficacy findings in the non-CCC cohort, Ray-Coquard says that these were better than anticipated, but that they may have been influenced by the inclusion of endometrioid cancers, which can be more indolent than CCC.
In the study, toxicities of grade 3 and above were reported in 47%, 41% and 39% of safety-evaluable patients in cohorts 1A, 2 and 3, respectively. The discontinuation rate due to adverse events was 10% or less in all cohorts.
Ray-Coquard notes that, “Currently, the data suggest that the main grade 3–4 toxicities are haematological, which are generally manageable in the clinic. However, the emergence of non-haematological malignancies, for example gastrointestinal side-effects, could be more problematic for patients.”
In terms of the way forward for ATR inhibitors in tumours with CCC histology, Ray-Coquard concludes that looking at PD-1 inhibition as a combination partner for ceralasertib could be a logical next step, as immune therapy is potentially active in these types of tumours.
Abstract discussed:
Banerjee S, et al. ATR inhibitor alone (ceralasertib) or in combination with olaparib in gynaecological cancers with ARID1A loss or no loss - results from the ENGOT/GYN1/NCRI ATARI trial. ESMO Gynaecological Cancers Congress 2023, Abstract 34O
Proffered Paper Session, 23.02.2023, h. 11:45 – 12:45, Auditorium 113. Watch the session on demand on the Congress virtual platform.