In advanced pre-treated endometrial cancer, patient-reported outcomes support efficacy benefits of the combination therapy
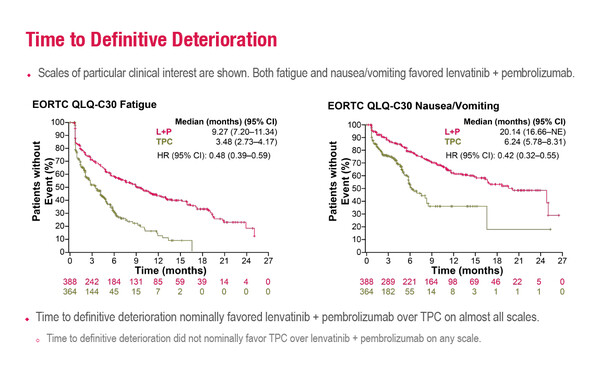
At a Mini Oral Session at the ESMO Gynaecological Cancers Congress 2022 today, a post-hoc analysis of the phase III Study 309/KEYNOTE-775 trial reports that lenvatinib plus pembrolizumab delays the time to deterioration of quality of life (QoL) in patients with advanced endometrial cancer whose disease had progressed on prior platinum-based therapy (Abstract 20MO). The combination therapy was associated with improved time to definitive deterioration (TTdD) in symptoms or functional status/global health status (GHS) compared with treatment of physician’s choice (TPC), with benefits observed in most of the scales assessed.
Patient-reported outcomes (PROs) and QoL information are gaining increasing attention in clinical studies to determine the right treatment for patients. In this analysis, PROs using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30, QLQ-Endometrial Cancer Module (QLQ-EN24) and EQ-5D-5L were assessed on day 1 of each treatment cycle until the end of treatment and results were analysed for patients receiving ≥1 study dose and providing ≥1 post-baseline PRO measurement. Time to first deterioration (TTfD) (for EORTC QLQ-C30 and QLQ-EN24) was defined as the time from treatment start to the first onset of a ≥10-point increase (symptom score) or decrease (functional/GHS score) from baseline. The definition for TTdD was the same, except that for this parameter, there could be no subsequent recovery.
“These findings are really reassuring and highlight the value of PROs in capturing the patient’s perspective of the impact that side effects and symptoms have on their quality of life, something that is particularly important for patients receiving non-curative treatment,” says Dr Ilaria Colombo from the Oncology Institute of Southern Switzerland (IOSI), Bellinzona, Switzerland. Lenvatinib plus pembrolizumab has become standard of care in second-line for patients with advanced endometrial cancer following the results of the 309/KEYNOTE-775 trial. This study demonstrated that the combination therapy significantly improved progression-free survival (PFS) and overall survival compared with TPC in patients with pre-treated advanced endometrial cancer, most of whom had mismatch repair-proficient disease (N Engl J Med. 2022;386:437–448). Despite a higher incidence of grade ≥3 adverse events with lenvatinib plus pembrolizumab, the study did not report a significant difference between the treatment arms in health-related quality of life (HRQoL) at 12 weeks (J Clin Oncol. 2021;39(15Suppl):5570–5570).
“We know from the primary analysis of this study that lenvatinib plus pembrolizumab was associated with a higher rate of dose reduction and treatment discontinuation due to side effects. Today’s results show that despite the greater toxicity, this did not lead to definitive deterioration in HRQoL, suggesting that at least some of the side effects were reversible,” says Colombo.
In the analysis presented, TTfD was generally similar between the two treatment arms for functional scales, although there were some between-arm differences in symptom scales: dyspnoea, poor body image, tingling/numbness and hair loss were better with lenvatinib plus pembrolizumab, whereas pain, appetite loss, diarrhoea and muscular pain were better with TPC. The mean observation period for PRO measures was usually longer for lenvatinib plus pembrolizumab than TPC.
According to Colombo, time on treatment is a factor that also needs to be taken into consideration: “Given the longer PFS achieved with lenvatinib plus pembrolizumab, it is perhaps not surprising that symptom deterioration would be delayed compared with TPC. However, this needs to be balanced alongside the increased chance of experiencing a side effect the longer a patient stays on treatment.”
Abstract discussed:
Lorusso D, et al. Time to deterioration in quality of life in patients (pts) with advanced endometrial cancer (aEC) treated with lenvatinib plus pembrolizumab (L+P) or treatment of physician’s choice (TPC). ESMO Gynaecological Cancers Congress 2022, Abstract 20MO
Mini Oral Session, 18.06.2022, h. 13:00 – 14:00, Auditorium 1A
Watch the session on demand on the Congress virtual platform.