Data from clinical trials show the value of liquid biopsy in treatment decision-making and prognostication
Data from two presentations at ESMO Gastrointestinal Cancers Congress 2024 (Munich, 26–29 June) highlight the clinical utility of liquid biopsies detecting circulating tumour DNA (ctDNA) in guiding treatment for patients with metastatic colorectal cancer (mCRC) and providing prognostic information post-surgery for patients with stage 3 colon cancer.
In the first study, the phase II CAPRI 2-GOIM trial, molecular assessments were performed at baseline using next-generation sequencing (NGS) on tumour tissue and plasma ctDNA in patients with RAS/BRAF wild-type mCRC (Abstract 6MO). Plasma ctDNA results were available for 206 patients, of whom 19 had molecular alterations associated with resistance to anti-EGFR therapies (18 patients with alterations in RAS and one patient with alterations in BRAF). Overall, 166 patients had both tissue and ctDNA results available. Among RAS-mutated tumours, high (94.6%) concordance was reported between tissue and ctDNA, except for mutations found in nine patients: three mutations found only in tissue and six mutations found only in ctDNA. Remarking on these results, Dr Pashtoon Murtaza Kasi from Weill Cornell Medicine, New York, NY, USA, says, “This trial contributes to a growing body of evidence supporting the use of ctDNA in patients with mCRC. It shows the clinical value of baseline plasma NGS, not only in guiding the selection of patients for anti-EGFR therapy but also in detecting other genetic aberrations that may otherwise have been missed.” However, he also highlights that, “Liquid biopsy is not necessarily a replacement for tissue biopsy but should instead be considered as a complementary tool.”
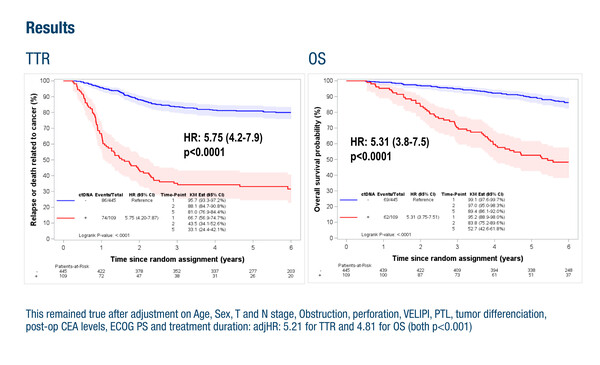
A second presentation showed the value of ctDNA as a prognostic marker in patients with stage 3 colon cancer who received surgery with curative intent (Abstract 7MO). In the IDEA-France (GERCOR) and IDEA-Greece (HORG) phase III trials, ctDNA was detected in 19.7% of 554 patients with available post-surgical blood samples and was shown to be an independent prognostic marker for time to recurrence (adjusted hazard ratio [HR] 5.21; p<0.001) and overall survival (adjusted HR 4.81; p<0.001). This analysis also examined the value of the Immunoscore® assay. Commenting on these results, Kasi highlights, “This is one of the first studies to evaluate the combination of ctDNA with Immunoscore, which was able to provide prognostic information for patients who are ctDNA negative but not ctDNA positive. These data support the prognostic value of ctDNA, particularly in the detection of minimal residual disease post-surgery.”
Looking ahead, Kasi notes that the next steps for these assays will be to establish practical utility, including assessing cost-effectiveness and integration into clinical workflow. “For instance, it will be important to determine whether all patients require both plasma and tissue testing or if it will be feasible to perform plasma testing first and then resort to tissue testing where necessary.” He concludes, “The combination of these tools offers a comprehensive approach to personalised cancer care looking at genetic, immune and dynamic markers of disease.”
Abstracts discussed:
Martini G, et al. Evaluation of plasma assessed comprehensive genomic profiling before first line treatment with FOLFIRI plus cetuximab in RAS/BRAFV600E wild type metastatic colorectal cancer patients in the CAPRI 2-GOIM trial. ESMO Gastrointestinal Cancers Congress 2024, Abstract 6MO
Mini Oral Session 1, 26.06.2024, h. 16:30 – 18:00, Room 14
Taieb J, et al. Combined analyses of ctDNA and Immunoscore in stage III colon cancer patients: a post hoc analysis of the IDEA-France and -Greece trials. ESMO Gastrointestinal Cancers Congress 2024, Abstract 7MO
Mini Oral Session 2, 28.06.2024, h. 16:30 – 17:45, Room 1