Results from the NorthStar trial highlight the potential for integrating local therapy with targeted agents to extend disease control in this setting
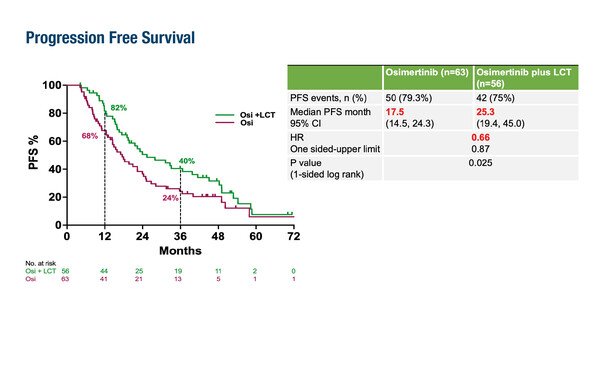
The addition of local consolidative therapy (LCT) to osimertinib improved progression-free survival (PFS) versus osimertinib alone in patients with EGFR-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC) in the NorthStar trial according to results presented at the ESMO Congress 2025 (Berlin, 17–21 October) (LBA72).
In the trial, patients with tyrosine kinase inhibitor (TKI) naïve EGFR exon 19 deletion (ex19del) or L858R, or acquired EGFR T790 mutation-expressing disease after first- or second-generation TKIs who had non-progressing disease after osimertinib induction therapy received either osimertinib plus LCT (n=56 [surgery: n=17; radiotherapy: n=33; both: n=6]) or osimertinib alone (n=63).
A small, single-arm, phase II study previously reported the potential benefits of adding localised radiotherapy alone to osimertinib in patients with EGFR-mutant metastatic NSCLC (EClinicalMedicine. 2025:87:103435). Dr Marina Garassino of the University of Chicago, USA, notes that the NorthStar trial builds on this premise and the strong single-agent activity of osimertinib, which typically achieves a PFS of 17–19 months. “Data from NorthStar are highly provocative, showing a 34% reduction in the risk of progression,” she says commenting on the PFS data – the trial’s primary endpoint – of 25.3 months with osimertinib plus LCT versus 17.5 months with osimertinib alone (hazard ratio [HR] 0.66; p=0.025). “They indicate that the combination of osimertinib and LCT represents another treatment option for our patients.”
The benefit of LCT on median PFS was observed across subgroups, including patients with more than three metastases at randomisation (20.7 months versus 15.9 months; HR 0.73; 95% confidence interval [CI] 0.45–1.20), those with an EGFR ex19del (39.8 months versus 22.8 months; HR 0.58; 95% CI 0.33–1.0) and patients with an EGFR L858R mutation (19.1 months versus 11.0 months; HR 0.60; 95% CI 0.31–1.16). Patients who received comprehensive LCT exhibited greater benefit than those who received partial LCT (median PFS 33.1 months versus 15.1 months; HR 0.42; 95% CI 0.19–0.90). There were three cases of LCT-specific grade 3 adverse events: one of pneumonitis and one each of arterial injury and empyema. “Treatment appears overall to be well tolerated, although the risk of long-term toxicities will need to be assessed,” notes Garassino.
Despite the small sample size and heterogeneity of the trial population, according to Garassino, the results not only demonstrate a clear role for consolidative therapy in this setting, but they also suggest its application for patients with other molecular alterations. “In the context of the evolving treatment landscape for EGFR-mutated NSCLC, this combination of a third-generation TKI and local consolidation is a valuable addition to treatment and may be particularly useful for improving the outcomes of patients who are not candidates for the addition of chemotherapy or amivantamab as first-line treatment,” she concludes. The NorthStar trial highlights the potential for integrating local therapy with targeted agents to extend disease control in EGFR-mutated metastatic NSCLC, reinforcing the need for further investigation to optimise patient selection and long-term management strategies.
Programme details
Elamin YY, et al. NorthStar: A phase II randomized study of osimertinib (OSI) with or without local consolidative therapy (LCT) for metastatic EGFR-mutant non-small cell lung cancer (NSCLC). ESMO Congress 2025 - LBA72