While a HER2-targeted bispecific antibody showed extended survival in pre-treated gastric cancers, TKI–immunotherapy combinations failed to confer a survival benefit
Findings from three phase III trials presented at the ESMO Congress 2025 (Berlin, 17–21 October) have highlighted both progress and ongoing challenges in the treatment of HER2-positive gastric or gastroesophageal junction carcinomas (GC/GEJC) and untreated metastatic oesophageal squamous cell carcinoma (SCC) or advanced GC/GEJC.
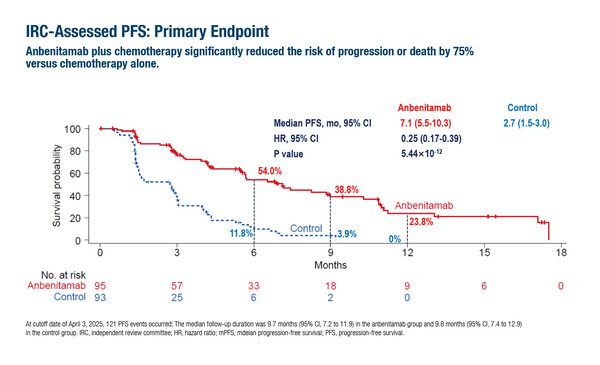
Interim analysis from the phase II/III KC-WISE trial of 188 patients with HER2-positive GC/GEJC who had failed previous therapy with a trastuzumab-based regimen showed that the addition of the novel HER2-targeted bispecific antibody, anbenitamab, to chemotherapy significantly improved median progression-free survival (mPFS; 7.1 months versus 2.7 months) and median overall survival (mOS; 19.6 months versus 11.5 months) – the co-primary endpoints – over chemotherapy alone (LBA78, key results in the box below). However, the combination was associated with a higher incidence of grade 3 or higher treatment-related adverse events. “These findings are encouraging,” says Dr Maria Alsina from Hospital Universitario de Navarra, Pamplona, Spain. “However, the study’s limited size and geographic restriction to Chinese centres will necessitate further international validation before a change to clinical practice is considered.” She also urged caution when interpreting the magnitude of benefit, pointing out that the performance of the control arm was worse than expected compared with other studies, such as the DESTINY-Gastric04 trial (N Engl. J Med. 2025;393:336–348). “This raises the possibility that efficacy with anbenitamab may have been overestimated,” she explains. “Nonetheless, these promising results with anbenitamab represent a step forward, and together with ongoing studies of other novel HER2-targeting therapies, they are likely to help shape the future treatment landscape for patients with HER2-positive GC.”
Less favourable results were reported from the phase III LEAP-014 study, where the addition of the TKI lenvatinib to pembrolizumab–chemotherapy did not improve mOS compared with the pembrolizumab–chemotherapy combination (17.6 months versus 15.5 months) in 850 patients with untreated metastatic oesophageal SCC, leading to termination of the study for futility (LBA79, key results in the box below). Previous interim analysis from the study had shown that the combination was tolerable with acceptable toxicity (Ann Oncol. 2023;34:S863). “These efficacy findings are consistent with the lack of OS benefit observed with this combination in the LEAP-015 trial in gastric adenocarcinoma (J Clin Oncol. 2025;43:2502–2514), despite preclinical models suggesting potential synergy between these treatments,” says Alsina. However, she adds, “It will be interesting to see subgroup analyses from LEAP-014 to see whether there are any potential benefits in PD-L1-positive subpopulations.”
Similarly, minor improvements in overall response and disease control rates were observed with the TKI regorafenib plus nivolumab in 462 patients with heavily pre-treated GC/GEJC in the phase III INTEGRATE IIb study (LBA80, key results in the box below). mOS was only 5.9 months with the combination compared with 6.3 months with investigator’s choice of chemotherapy, while mPFS was 1.9 months in both treatment arms. The rationale for INTEGRATE IIb came from the promising antitumour activity and manageable toxicity observed with this combination in the phase Ib REGONIVO study (J Clin Oncol. 2020;38:2053–2061) study. These results are disappointing given the poor prognosis of patients with GC/GEJC and the urgent need for effective treatment options following two lines of systemic treatment.
Given the heterogeneity of GI cancers, according to Alsina the future of treatment lies in the development of biomarker-guided therapies. “Such precision-guided strategies may allow us to design chemotherapy-free regimens, minimise unnecessary toxicity and ultimately deliver long-term clinical benefits in this difficult-to-treat and highly diverse group of diseases.”
At a glance:
Xu J, et al. KN026 in combination with chemotherapy for previously treated HER2 positive gastric or gastroesophageal carcinomas (GC/GEJC): Interim analysis of KC-WISE. ESMO Congress 2025 - LBA78
- N=188 (anbenitamab + CT n=95; CT: n=93)
- Anbenitamab + CT vs CT:
- mPFS 7.1 mo vs 2.7 mo; HR 0.25; p<0.00001
- mOS: 19.6 mo vs 11.5 mo; HR 0.29; p<0.00001
- IRC-ORR: 55.8% vs 10.8%; mDoR: 8.2 mo vs 2.9 mo
- Grade ≥3 TEAEs: 60.6% vs 51.6%
- TEAEs leading to death: 1 vs 8
Sun J-M, et al. Lenvatinib plus pembrolizumab and chemotherapy vs pembrolizumab and chemotherapy in untreated metastatic esophageal squamous cell carcinoma: The randomized phase 3 LEAP-014 study. ESMO Congress 2025 - LBA79
- N=850 (lenvatinib + pembrolizumab + CT n=423; pembrolizumab + CT: n=427)
- Lenvatinib + pembrolizumab + CT vs pembrolizumab + CT:
- Median follow-up: 22.1 mo (ITT population, IA2)
- mOS: 17.6 mo vs 15.5 mo; HR 0.92; p=0.1852
- mPFS: 7.2 mo vs 6.9 mo; HR 0.89
- ORR: 62.2% vs 54.8%; mDoR: 8.1 mo vs 6.8 mo
- Grade ≥3 AEs: 81.2% vs 79.1%
Goldstein D, et al. Regorafenib plus nivolumab vs investigator’s choice of chemotherapy in previously treated gastric or gastroesophageal cancer: INTEGRATE IIB, a randomized Phase 3 AGITG Intergroup [NHMRC-CTC/IKF/AIO, ACCRU, TCOG/NHRI] study. ESMO Congress 2025 - LBA80
- N=462 (regorafenib–nivolumab n=309; CT: n=153)
- Regorafenib–nivolumab vs CT:
- mOS: 5.9 mo vs 6.3 mo; HR 0.88; p=0.23
- mPFS: 1.9 mo vs 1.9 mo; HR 0.85
- ORR: 7.4% vs 2.6%; mDoR: 9.5 mo vs 6.3 mo
- Grade ≥3 AEs: 70.0% vs 49.3%