Early proof-of-concept is provided showing pan-tumour activity of a novel antibody–drug conjugate
Tumour-associated mucin-1 (TA-MUC1), a transmembrane glycoprotein, is an emerging anticancer target since it is widely expressed in most epithelial cancers but has limited expression in normal tissue. At the ESMO Congress 2025 (Berlin, 17–21 October), encouraging results were presented from a phase I/II, first-in-human study of DS-3939, a novel, first-in-class TA-MUC1-directed antibody–drug conjugate (ADC), which previously demonstrated TA-MUC1-dependent inhibition of cell growth in preclinical studies (Mol Cancer Ther. 2025;Jul 10) (Abstract 917O).
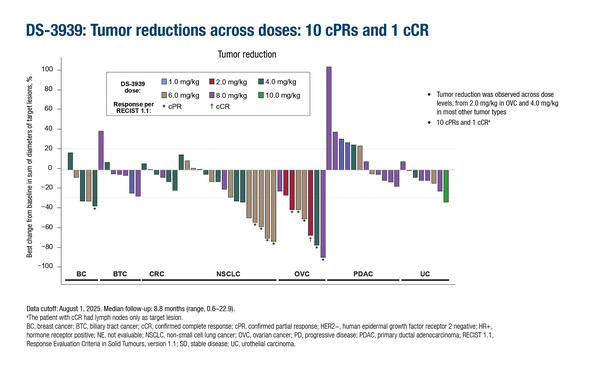
The dose escalation portion of DS-3939 comprised 64 patients with epithelial-type advanced solid tumours expected to overexpress TA-MUC1, including biliary, breast, non-small cell lung cancer (NSCLC), and ovarian, pancreatic and urothelial cancers. At a median follow-up of 8.8 months, a confirmed complete response was reported in 1 patient with ovarian cancer (in whom lymph nodes were the target lesion), while confirmed partial responses were reported in 10 patients with NSCLC, ovarian cancer and breast cancer. There were 39 patients in whom the best overall response was stable disease, and 12 had progressive disease. Dr Daniel Tan from the National Cancer Centre Singapore and Duke-NUS Medical School, Singapore, comments, “This is an exciting trial, designed to demonstrate proof-of-concept in terms of how therapeutically tractable MUC1 is as a tumour-associated antigen. The population was heavily pre-treated; patients had received a median of three prior lines of therapy, and more than one-third had previously received at least four lines of therapy for locally advanced or metastatic disease. Considering this, it is striking that tumour reductions were observed across the dose levels, some of which lasted more than 12 months. This bodes well for evaluation in later-phase clinical studies.”
The toxicity profile appeared manageable; overall, 34.4% of patients experienced treatment-related grade ≥3 adverse events (AEs), and 17.2% of patients discontinued study treatment due to treatment-emergent AEs. Seven cases of treatment-related interstitial lung disease (ILD) were reported, including one case of grade 3 ILD, and there was one treatment-related death. “DS-3939 has a deruxtecan (DXd) payload, so we might expect a similar side-effect profile to other established ADCs with this payload, such as trastuzumab-DXd and datopotamab-DXd,” surmises Tan.
Equally important to establishing the activity of a novel therapy and demonstrating proof of concept is the ability to select patients who are most likely to derive clinical benefit. At the same Proffered Paper session, results were presented for monotherapy with the MET-targeting ADC, telisotuzumab adizutecan (ABBV-400; Temab-A) in a biomarker-selected population of 100 patients with 19 types of pre-treated MET-amplified advanced solid tumours (Abstract 918O). A 46% objective response rate was reported across all dose levels and tumour types, with higher rates observed in patients with NSCLC (69%) and gastroesophageal adenocarcinomas (71%). Median progression-free survival was 8.5 months in the overall population at a median follow-up of 14.4 months.
“This ADC had previously been validated in specific tumour types, including EGFR-mutated NSCLC (J Clin Oncol. 2025;43:8512–8512) and colorectal cancer (J Clin Oncol 2025;43:258–258). However, the current analysis confirms that activity can be demonstrated across a diverse range of solid cancers using a different biomarker-selected population,” says Tan.
In summary, as our clinical experience with different targets, linkers and payloads continues to expand, each clinical trial of a novel ADC and/or biomarker provides additional insights on how best to leverage this exciting therapeutic modality. These data will fuel ongoing efforts to further optimise patient selection and drug design, ultimately fulfilling the promise of high precision ADCs.
Programme details:
Patel MR, et al. DS-3939, a tumor-associated mucin 1 (TA-MUC1)–directed antibody–drug conjugate (ADC), in patients (pts) with advanced/metastatic (adv/met) solid tumors: Initial results from a first-in-human (FIH) study. ESMO Congress 2025 - Abstract 917O
Murciano-Goroff YR, et al. Telisotuzumab adizutecan (ABBV-400; Temab-A) in patients with MET-amplified (MET-amp) advanced solid tumors: Results from a phase 1 study. ESMO Congress 2025 - Abstract 918O